2021 Volume 38 Issue 1 Pages 1-5
2021 Volume 38 Issue 1 Pages 1-5
Type I collagen, which consists of two α1 and one α2 chains, is an abundant extracellular matrix (ECM) protein. Changes in type I collagen are often associated with various diseases including fibrosis, osteogenesis imperfecta (OI), and Ehlers-Danlos syndrome (EDS). In the present study, we developed a method for quantification of type I collagen α1 (COL1A1) in a culture medium of LX-2 human hepatic stellate cells by using nano-liquid chromatography tandem mass spectrometry (nano-LC/MS/MS). After selecting a specific peptide of COL1A1, unlabeled and stable isotope-labeled peptides were used for quantitative analysis of COL1A1 by nano-LC/MS/MS. The concentration of secreted COL1A1 in the LX-2 cell culture medium was 38.78 ng/mL. The results indicate that this method is useful for quantifying COL1A1 in a cell culture medium.
Extracellular matrix (ECM) plays essential roles in all tissues and organs. Collagens consisting of Glycine-X-Y triplets are the major matrix proteins in connective tissues, and the proline residues at the Y position are hydroxylated to 4-hydroxyproline. There are at least 28 collagen types in vertebrates, and type I collagen is the most abundant collagen [ 1]. Type I collagen consists of two α1 (COL1A1) and one α2 (COL1A2) chains and it plays a crucial role in maintaining normal tissue structure and cell function [ 2]. Changes in type I collagen are associated with various diseases including fibrosis [ 2], diabetes [ 3], osteoporosis [ 4], cancers [ 5], osteogenesis imperfecta (OI) [ 6], and Ehlers-Danlos syndrome (EDS) [ 7]. Therefore, the development of a method for quantification of type I collagen would be helpful for the evaluation and diagnosis of collagen-related diseases.
Liquid chromatography tandem mass spectrometry (LC/MS/MS) has been used for qualitative and/or quantitative analyses of many proteins [ 8]. Although LC/MS/MS has been used for identifying collagens, quantification of types of collagen in biological samples using LC/MS/MS has rarely been performed [ 9]. Among the quantification methods using LC/MS/MS, the stable isotope labeling by amino acid in cell culture (SILAC) method has been used for the quantification of type I and type III collagens [ 10].
We have established a method for quantification of the serum form of tenascin-X (sTNX) in human serum by using nano-LC/MS/MS [ 11]. In this method, synthetic unlabeled and stable isotope-labeled peptides were used to quantify sTNX.
In the present study, we selected the peptides and established a method for quantification of COL1A1 in a culture medium of LX-2 human hepatic stellate cells by using nano-LC/MS/MS. This method could be used for analyses of other types of collagen.
Materials and reagents
Type I collagen purified from human placenta, pepsin and tris (hydroxymethyl) aminomethane (Tris) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Synthetic unlabeled and stable isotope-labeled GVVGLP*GQR peptides (P* indicating 4-hydroxyproline) were purchased from GL Biochem (Shanghai, China) and CS Bio (Menlo Park, CA, USA), respectively. The stable isotope-labeled peptide had an isotope-labeled [13C6, 15N4]-arginine residue at its C-terminus. Trifluoroacetic acid (TFA), hydrochloric acid (HCl), sodium chloride (NaCl), acetic acid, penicillin and streptomycin, and L-ascorbic acid were purchased from Wako (Osaka, Japan). Trypsin was obtained from AB Sciex (Foster, CA, USA). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The LX-2 human hepatic stellate cell line was purchased from Merck Millipore (Burlington, MA, USA). A Spin-X UF concentrator was from Corning (Corning, NY, USA). A BCA protein assay kit was from Thermo Fisher Scientific (Waltham, MA, USA).
Cell culture and extraction of collagen from the cell culture medium
LX-2 cells were seeded at a density of 1.0×106 or 1.5×106 cells per 100 mm dish and cultured for 2 or 3 days until 100% confluency in 10 mL of DMEM containing 2% FBS supplemented with 50 units/mL of penicillin and 50 μg/mL of streptomycin at 37℃ in a humidified 5% CO2 incubator. Thereafter, the medium was changed to 10 mL of DMEM with 0.5% FBS and 200 μM L-ascorbic acid and the cells were cultured for a further 2 days. The collected culture medium was digested with 0.5 mg/mL of pepsin in 0.1 N HCl for 16 h at 4℃. To extract collagens from the culture medium, salt precipitation was performed with 1.2 M NaCl for 3 h at 4℃. Collagens were precipitated by centrifugation at 30,000 g for 30 min at 4℃. The precipitate was dissolved in 5 mM acetic acid. The solution was concentrated by using a Spin-X UF concentrator and the protein concentration was measured by a BCA assay.
Trypsin digestion
A sample was neutralized with 0.1 M Tris-HCl (pH 7.4) and 0.4 M NaCl and then denatured at 60℃ for 30 min. The sample was then digested with trypsin at a 10:1 (protein : trypsin ratio) at 37℃ for 16 h.
Nano-LC/MS/MS
COL1A1 peptides in the digests from three or four independent samples were analyzed three times with Pinpoint software by using nano-LC/MS/MS (Thermo Fisher Scientific, Waltham, MA, USA) as described previously [ 11]. Data are means ± standard deviation (SD).
In order to select the COL1A1 peptide for quantification of COL1A1 in the LX-2 cell culture medium, commercially available type I collagen purified from human placenta used as a standard material was digested with trypsin and the product peptides were analyzed with Pinpoint software by using nano-LC/MS/MS as described previously [ 11]. Among the candidate peptides that were tested for quantification of COL1A1, peptide GVVGLP*GQR showed stronger intensity than that of other peptides. Therefore, peptide GVVGLP*GQR was synthesized and analyzed by using nano-LC/MS/MS. The transitions and the retention time of peptide GVVGLP*GQR from human placenta type I collagen were identical to those of synthetic peptide GVVGLP*GQR ( Fig. 1A and 1B). However, a homology search revealed that the amino acid sequence of human COL1A1 peptide GVVGLP*GQR (UniProt accession No. P02452, aa 959-aa 967), was identical to that of bovine COL1A1 peptide, GVVGLP*GQR (UniProt Accession No. P02453, aa 958-aa 966). Therefore, we measured peptide GVVGLP*GQR in a cell-free control medium as a background level since the culture medium contains 0.5% FBS as well as in the LX-2 cell culture medium by using nano-LC/MS/MS. The transitions and the retention time of peptide GVVGLP*GQR from the LX-2 cell culture medium were also identical to those of synthetic peptide GVVGLP*GQR ( Fig. 1C). Subsequently, quantification of COL1A1 in the control medium and in the LX-2 cell culture medium was performed by calculating the peak area ratio of the unlabeled and stable isotope-labeled peptides. The concentrations of COL1A1 in the control medium and LX-2 cell culture medium were 1.44 ± 0.45 ng/mL and 40.22 ± 10.71 ng/mL ( Table 1), respectively. Thus, based on the result of subtraction of the concentration of COL1A1 in the control medium from that in the LX-2 cell culture medium, the concentration of secreted COL1A1 in the LX-2 cell culture medium was 38.78 ng/mL. These results indicate that the present method using peptide GVVGLP*GQR with nano-LC/MS/MS is useful for quantification of COL1A1 in a cell culture medium.
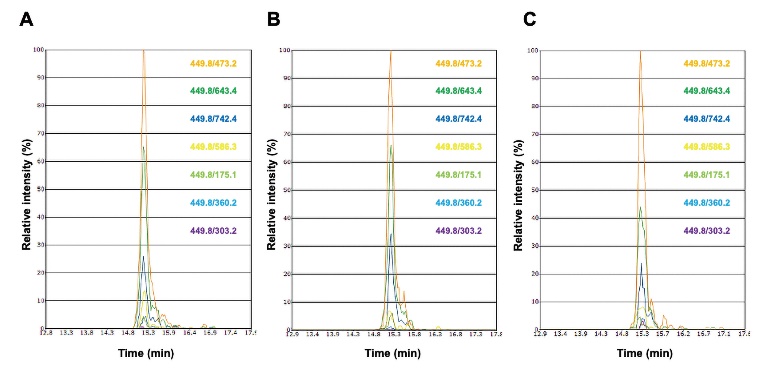
Transitions and retention times of peptide GVVGLP*GQR. Peptide GVVGLP*GQR from commercially available type I collagen purified from the human placenta (A), synthetic peptide GVVGLP*GQR (B), and peptide GVVGLP*GQR in the culture medium from LX-2 human hepatic stellate cells (C) were analyzed by nano-LC/MS/MS. The transitions of peptide GVVGLP*GQR are shown on the right side of each figure. Vertical line indicates relative intensity, whereas horizontal line indicates retention time.

Concentrations of COL1A1 in cell-free control medium and LX-2 human hepatic stellate cell culture medium
In the present study, we established a method for quantifying COL1A1 by using nano-LC/MS/MS. While the LC/MS/MS method has been used for identification of collagens, quantification of collagens by LC/MS/MS has not been investigated in detail [ 9, 12]. Furthermore, it is conceivable that accurate quantification by LC/MS/MS is impaired by suppression of ionization by matrix components in biological samples such as plasma [ 13]. Therefore, in order to overcome these faults and to establish a method for accurate quantification of peptides by LC/MS/MS, stable isotope-labeled internal standard peptides have been used to quantify bioactive peptides in plasma [ 14].
Taga et al. [ 10] developed a method for quantification of type I collagen using SILAC. In that method, stable isotopically heavy lysine, arginine, and proline were added to the culture medium metabolically to produce stable isotope-labeled collagen (SI-collagen) for mass spectrometric analysis. After purification of SI-collagen from the cell culture medium, type I collagen from rat tissues mixed with the SI-collagen was digested with trypsin and then type I collagen was quantified by using the SI-collagen peptides as internal standards. However, this method requires living cells, optimization of labeling, and purification and digestion procedures to obtain the SI-collagen peptide [ 10, 15].
On the other hand, our method initially searched for candidate collagen peptides including hydroxyproline for mass spectrometric analysis by using Pinpoint software. The peptides for quantification of type I collagen were selected using the trypsin digests of type I collagen from human placenta by nano-LC/MS/MS, because the proline residues of placenta type I collagen are post-translationally hydroxylated. Then, the specific stable isotope-labeled collagen peptide was synthesized to quantify type I collagen. In the present study, by using the stable isotope-labeled peptide GVVGLP*GQR as an internal standard, we measured the concentration of COL1A1. The concentration of secreted COL1A1 in the LX-2 cell culture medium was calculated to be 38.78 ng/mL. Remarkably, Liu et al. [ 16] reported the concentration of type I collagen in a conditioned medium from LX-2 cells measured by an enzyme-linked immunosorbent assay (ELISA). The concentration of COL1A1 (approximately 17.75 ng/mL) estimated from that of type I collagen (26.3 ng/mL) measured by ELISA [ 16] was similar value to that obtained in our study using the nano-LC/MS/MS method (38.78 ng/mL). Thus, we consider that our method is reliable for quantification of COL1A1 in a cell culture medium.
In conclusion, we developed a method for quantification of COL1A1 by using nano-LC/MS/MS. This method can be used for quantifying other types of collagens in a cell culture medium. A study is now underway to improve the method for simultaneously quantifying type III and type V collagens together with type I collagen. Such a method would be useful for diagnosing collagen-involved diseases such as vascular and classical EDS, the causal proteins of which are type III and type V collagens, respectively.
Conflict of interest
All authors have no conflicts of interest.