Abstract
Depression is one of the most common mental disorders. Glial cells have been reported to play a role in the pathogenesis of depression. Meanwhile, Ninjin’yoeito (NYT), a Kampo medicine, is a multi-component drug that was reported to improve depressive symptoms in patient and animal models. In addition, some of its components have an effect on glial cells. However, the mechanism remains unclear. The study’s aim was to investigate the effect of NYT in recovering depressive-like behavior and glial pathological changes in the hippocampus induced by lipopolysaccharide. Here, we showed that NYT improved the forced swim test performances of the rat. Furthermore, NYT also inhibited microglial activation in the hippocampus, but the results were different in astrocytes. These results suggest that NYT alleviates depressive-like behavior, and the effect may be associated with the attenuation of glial cell activation.
INTRODUCTION
Depression is the most common psychiatric disorder [1–4]. Characteristic symptoms of depression include a sad mood, despair, anhedonia, and in cases of severe depression found suicide [5–7]. Post mortem studies suggested that glial pathological changes were involved in the hippocampus of depressed patients [8–11]. Meanwhile, some animal studies also reported that activation of glial cells occurs in animal models of depression [12–14]. Therefore, it is pointed out that the activation of glial cells may play a role in the pathogenesis of depression [15]. In addition, lipopolysaccharide (LPS) is one of the most commonly used as an active inflammatory substance to induce depressive-like behavior due to the glial cell activation in animal studies [16–19].
Ninjin’yoeito (NYT), a prescription derived from Chinese traditional medicine, it is composed of 12 crude herbs: poria sclerotium, ginseng, astragalus root, rehmannia root, atractylodes root, angelica root, peony root, cinnamon bark, citrus unshiu peel, polygala root, glycyrrhiza, and schisandra fruit in a Japanese herbal medicine formula (Table 1) [20, 21]. In clinical studies reported that NYT with donepezil improved depressive symptoms in Alzheimer’s disease (AD) patients [22]. In addition, NYT could reduce the symptoms of apathy in AD patients [23]. In the animal depression model, NYT improved depressive-like behavior induced by the chronic corticosterone administration [24]. Furthermore, several studies reported that some active ingredients of NYT such as ginseng, polygala root, or angelica root suppressed glial cell activation [25–28]. These results suggest the potentiality of NYT in the suppression of glial cell activation-related depression behavior.
In the present study, we investigated the effect of NYT treatment on depressive-like behavior and activated glial cells induced by LPS. We hypothesized that NYT might alleviate depressive-like behavior and attenuate microglial and astrocyte activation in the rat depression model. First, we conducted a forced swim test (FST) as a despair behavior model [29], which is one of the most commonly used tests for animal depressive-like behavior studies [30, 31]. Next, we examined the activation of microglia and astrocytes in the hippocampus of the rat depression model. Microglia and astrocytes are the most important glial cells as the inflammation response and innate immunity in the central nervous system [32–36].
Table 1.
The composition of ninjin’yoeito. Each 9.0 g of ninjin’yoeito extract contains 6.0 g of a dried extract consisting of the below drugs
| The Component’s Name of Crude Drug
|
Formula of NYT*
(g)
|
| Glycyrrhiza |
1.0 (3.22%) |
| Ginseng |
3.0 (9.67%) |
| Peony Root |
2.0 (6.45%) |
| Japanese Angelica Root |
4.0 (12.90%) |
| Polygala Root |
2.0 (6.45%) |
| Poria Sclerotium |
4.0 (12.90%) |
| Cinnamon Bark |
2.5 (8.06%) |
| Atractylodes Rhizome |
4.0 (12.90%) |
| Schisandra Fruit |
1.0 (3.22%) |
| Citrus Unshiu Peel |
2.0 (6.45%) |
| Astragalus Root |
1.5 (4.83%) |
| Rehmannia Root |
4.0 (12.90%) |
*NYT, ninjin’yoeito
MATERIALS AND METHODS
Animals
Wistar male rats, eight-week-old (Japan SLC, Inc., Shizuoka, Japan), were used in the current study. Animals were housed in groups under standard conditions with 12 h light/12 h dark cycle (lights on from 7:00 to 19:00), a temperature of 23 ± 2℃ with a humidity level of 55 ± 5%, and free access to food and water. Handling procedures were performed to reduce the experimental stress for one week before the experiment. The experimental protocol was reviewed and approved by the Shimane University Animal Ethics Committee under the guidelines of the National Health and Medical Research Council of Japan (Authorization No: IZ2-104).
Drugs
Ninjin’yoeito (NYT; TJ-108, Lot no. 2180108010) was supplied by Tsumura & Co., Tokyo, Japan, in dry powder form and dissolved using distilled water. A dose-escalation trial was performed to determine the dose of NYT to be used (unpublished data), and the dose range was based on reports of previous animal experiments [24, 37]. Five different doses were tested (500 to 10,000 mg/kg body weight/day). Furthermore, we observed the influence on behavior tests and side effects such as weight loss, diarrhea, and vomiting [38, 39]. Finally, the dosage of 2000 mg/kg body weight/day was selected for use in this study.
Lipopolysaccharide (Escherichia coli serotype 0111:B4; Sigma-Aldrich) was dissolved with saline before use. The dosage of LPS was determined based on preliminary study results of the LPS effect on microglia activation (unpublished data); the doses of 0.1, 0.5, and 1 mg/kg body weight every other day were chosen in accordance with previous reports showing that these doses were effective in inducing depressive-like behavior in animals [40–42]. Finally, the preliminary study results indicated dosage of 1 mg/kg body weight every other day.
Experimental Protocol
The rats were divided into four groups (n = 6): sham, NYT, LPS, and NYT-LPS. NYT or distilled water as control was administered orally by intragastric gavage once daily in the morning (8:00 to 11:00) from day 1 to day 16 (Fig. 1). LPS or saline as control was administered after NYT via intraperitoneal injection every other day for a total of eight times. Meanwhile, body weight was monitored and recorded every day before drug administration. Furthermore, habituation of the forced swim test was performed on day 16 before drug administration and the real test was performed on the next day. Next, brain samples were collected immediately after completing the behavioral tests for immunohistochemistry analysis.
FST
Depressive-like behavior was analyzed by immobility time in the FST. This behavioral test measures the rat’s efforts to escape from the water cylinder, but, consequently will show immobility that may resemble a behavioral despair [31]. The FST was performed as previously reported with some modifications [13, 31, 43]. Briefly, each rat was placed individually and gently in a plastic cylinder (diameter 19 cm) filled with 10 L water (temperature 25 ± 2℃, depth 40 cm) for two consecutive days. The rat was unable to touch the top and bottom of the cylinder. After completing each trial, the rat was dried with a towel and placed in an incubator to prevent hypothermia, then returned to the home cage. In addition, the cylinder was washed, dried, and refilled water to avoid impact on subsequent animals. The rat was gently placed in water for 15 minutes as habituation on the first day. The next day, the rat was gently placed in the water and its behavior was recorded on a video camera for 6 minutes. The immobility time that occurred during the test was calculated manually by a blind trained observer. Rats was considered immobile when they remain afloat without struggling, less moving, and they move only to keep their heads above the surface of the water.
Brain Section Preparation
Brain sections were prepared as described previously with some modifications [13]. In brief, we used a mixture of three drugs: 2 mg of midazolam (Dormicum, Astellas Pharma, Tokyo, Japan), 0.15 mg of medetomidine (Domitor, Nippon Zenyaku Kogyo, Tokyo, Japan), and 2.5 mg/kg body weight of butorphanol (Vetorphale, Meiji Seika Pharma, Tokyo, Japan), and 0.9% saline (Otsuka Pharmaceutical Factory, Tokushima, Japan) was added to adjust the mixture to 0.5 mL/100 g body weight to induce deep anesthesia via intraperitoneal injection. The rats were perfused with 500 mL of physiological saline, followed by 500 mL of 4% paraformaldehyde in phosphate buffer solution (Fujifilm, Wako, Osaka, Japan) via transcardial. The brains were shortly taken and post-fixed with 4% paraformaldehyde in phosphate buffer solution at room temperature (RT) for 4 h. Next, the brains were immersed in 10% sucrose solution at 4℃ overnight and were subsequently immersed in 20% sucrose solution at 4℃overnight. Then, the brains were put in the frontal plane and cut into 40-μm thickness slices using a freezing microtome (Microm HM 430; Thermo Scientific, Germany).
Immunohistochemistry of Glial Markers
Meanwhile, to evaluate the glial cell activation, we used ionized calcium-binding adaptor molecule 1 (Iba1) for activated microglial cells [44] and glial fibrillary acidic protein (GFAP) for activated astrocytes cells [45]. Immunohistochemical staining was conducted as described previously with some modifications [13, 46]. Free-floating brain tissue sections were incubated in 1% H2O2 for 30 min at RT. Subsequently, tissue sections were incubated with 0.2% Triton-X and 1.5% normal horse serum in 0.1 M phosphate buffer for 1 h at RT. Then, the sections were incubated with the following primary antibodies: rabbit anti-Iba1 (1:2000, Wako, Osaka, Japan) and goat anti-GFAP (1:2000, Abcam plc., Cambridge, UK) overnight. Next, the sections were incubated with biotinylated anti-rabbit (or anti-goat) IgG antibody (1:1000, standard ABC kit, Vector Laboratories, Inc., CA, USA) for 1 h at RT. Furthermore, the sections were incubated with an avidin-biotin-peroxidase complex in phosphate-buffered saline (PBS) for 1 h. The sections were incubated in PBS containing 0.1% H2O2 and 0.5% diaminobenzidine (DAB) to promote immunoreactivity for 10 min. Then, the sections were rinsed with PBS for 30 minutes to stop the DAB reaction. Finally, the sections were mounted onto gelatin-coated slides. And, the sections were immersed in graded alcohol solutions for dehydration. Subsequently, the sections were covered with mounting medium and a coverslip (Matsunami Glass, Osaka, Japan).
Immunohistochemistry Image Analysis
Pure black and white analysis were performed to measure microglia and astrocyte immunoreactivity intensity via DAB staining as described previously with some modifications [47]. A pure black and white analysis were conducted using ImageJ software (ImageJ 1.52a, National Institutes of Health, MD, USA). DAB images were captured with a 20x objective lens using the BZ-X700 all-in-one microscope (Keyence, Osaka, Japan) at three areas of interest in the hippocampal formation, the dentate gyrus (DG), cornu ammonis (CA) 1, and CA3 regions. Specific areas were traced and captured manually using ImageJ software for analysis by a trained observer in a blinded manner. Twenty images were obtained bilaterally from each area, and in total, 60 images were analyzed from each rat. All immune-labeled elements were automatically converted beyond the threshold range to pure black pixels, and then the remaining image area was converted to pure white pixels (Fig. 2). The percentage of pure black pixels was calculated automatically by the software for statistical analysis.

Statistical Analysis
All data were presented as the mean ± standard error of the mean (S.E.M.). We use a one-way analysis of variance followed by a post hoc Tukey’s honestly significant difference test to evaluate the differences among groups. The statistical analyses were performed with SPSS software (IBM SPSS Statistics for Windows Version 23, SPSS Japan Inc., Tokyo, Japan), and the p-value was statistically significant when less than 0.05. All figures were prepared using Microsoft Excel.
RESULTS
Basic Characteristics (Body Weight Profiles)
Body weight was monitored as a dose adjustment of drug administration and evaluation of the rat’s physiological response to stress [48]. The average body weight was 295.46 ± 9.26 g in the sham, 294.27 ± 3.00 g in the NYT, 255.85 ± 9.31 g in the LPS, and 281.24 ± 7.59 g in the NYT-LPS groups after 16 days of drug administration. These findings suggest that LPS inhibited weight gain, but, NYT did not significantly recover it.
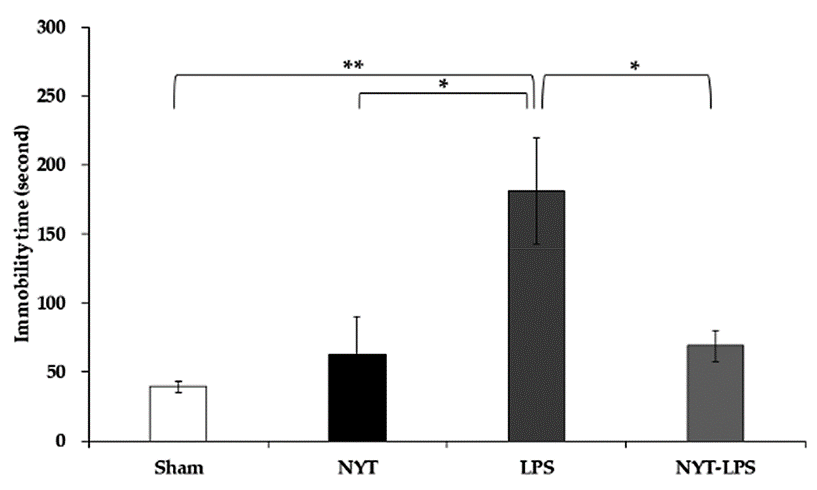
Effect of Treatment with NYT on Immobility Time in the Forced Swim Test
Immobility time increased significantly in the LPS (181.17 ± 38.28 s) group compared to the sham (39.17 ± 4.29 s) and NYT (63.00 ± 27.02 s) groups (Fig. 3). Furthermore, the NYT-LPS (68.83 ± 11.39 s) group shows a significant decrease in immobility time compared to the LPS group (Fig. 3). These findings suggest that NYT improves LPS-induced depressive-like behavior.
Effect of NYT on Microglial Activation in the Hippocampus
Microglial activation was examined in three different hippocampus areas: the DG, CA1, and CA3. In the DG area, representative photomicrograph images showed a higher expression level of Iba1 immunoreactivity in the LPS group than sham and NYT. Meanwhile, LPS-induced Iba1 expression levels were decreased with NYT administration (Fig. 4A). Quantification analysis showed that Iba1 immunoreactivity was increased significantly in the LPS group compared to sham and NYT. At the same time, the NYT-LPS group showed a significant decrease compared to the LPS group (Fig. 5A).
For the CA1 region, representative photomicrographs showed a higher expression level of Iba1 immunoreactivity in the LPS group than sham and NYT. However, there has no difference between the NYT-LPS and LPS groups (Fig. 4B). The analysis result showed that Iba1 immunoreactivity was increased significantly in the LPS group compared to sham and NYT. However, no significant difference was found between the NYT-LPS and the LPS groups (Fig. 5B).
In the CA3 regions, representative photomicrographs showed a higher expression level of Iba1 immunoreactivity in the LPS group than sham and NYT. At the same time, no differences were found between the NYT-LPS and LPS groups (Fig. 4C). Quantification results showed significantly increased Iba1 immunoreactivity in the LPS group compared to the sham and NYT. However, there was no significant difference between the NYT-LPS and the LPS groups (Fig. 5C). These findings suggest that NYT attenuates microglial activation in the hippocampus, especially in the DG region, of the LPS-induced depression model.
Quantification analysis of ionized calcium-binding adaptor molecule 1 (Iba1) immunoreactivity was done as described in the Materials and Methods section. Analysis results of the dentate gyrus (DG) (A), cornu ammonis 1 (CA1) (B), and CA3 (C) regions were shown. Four groups of rats were used, and each group had six rats. Each value expressed the mean ± S.E.M. Statistical significance is shown as follows: *p < 0.05, **p < 0.005, n.s., not significant.
Effect of NYT on Astrocytes Activation in the Hippocampus
Astrocytes activation also was examined in three different hippocampus areas: the DG, CA1, and CA3. In the DG area, representative photomicrograph images showed a higher expression level of GFAP immunoreactivity in the LPS group than sham and NYT. However, there has no difference between the NYT-LPS and LPS groups (Fig. 6A). Quantification analysis showed that GFAP immunoreactivity was increased significantly in the LPS group compared to sham and NYT. Meanwhile, the NYT-LPS group showed no significant difference compared to the LPS group (Fig. 7A).
In the CA1 region, representative photomicrographs showed a higher expression level of GFAP immunoreactivity in the LPS group than sham and NYT. However, there has no difference between the NYT-LPS and LPS groups (Fig. 6B). The quantification analysis result showed that GFAP immunoreactivity was increased significantly in the LPS group compared to sham and NYT. However, no significant difference was found between the NYT-LPS and the LPS groups (Fig. 7B).
In the case of CA3 region, representative photomicrographs showed a higher expression level of GFAP immunoreactivity in the LPS group than sham. At the same time, no differences were found in the LPS group compared to the NYT and NYT-LPS groups (Fig. 6C). Quantification results showed significantly increased GFAP immunoreactivity in the LPS group compared to the sham. However, there has no significant difference between the LPS group with the NYT and NYT-LPS groups (Fig. 7C). Our findings suggest that LPS-induced astroglial activation in three different areas of the hippocampus, and, NYT did not recovered it.
DISCUSSION
NYT medicinal herb consists of twelve crude drug components, and one of the functions is as anti-inflammatory [20, 49, 50]. NYT and its components have been reported to penetrate the blood-brain barrier [51] and maintain adequate cerebral blood flow [52]. Therefore, we hypothesized that NYT might ameliorate LPS-induced depressive behavior and glial cell immunoreactivity.
The present study showed that NYT improves the immobility time in the FST in animal models of LPS-induced depression. These findings suggested that NYT improves depressive-like behavior (despair behavior). A previous study reported that NYT administration improved the immobility time of C57BL/6 mice to chronic administration of corticosterone in the FST and the tail suspension test [24]. Among the component of NYT, gomisin N, the active substance of schisandra fruit, was reported to ameliorate the performance of male ddY mice in the FST by attenuating inflammation in the hypothalamic paraventricular nucleus and central nucleus of the amygdala [53]. Based on these findings, it can be speculated that NYT or its components may improve hippocampal neuroinflammation in animal models of LPS-induced depressive-like behavior, which may recover despair behavior.
It is well evidenced that glial cell activation caused neuroinflammation in animal model of depression [12–14]. Interestingly, we found that NYT treatment significantly reduced Iba1 expression in the hippocampal DG, suggesting that NYT attenuates microglial activation induced by LPS. To the best of our knowledge, this is the first study to investigate the effects of NYT on microglia pathological changes in depression animal models. Indeed, another active component of NYT, tenuigenin (the active substance of polygala root), inhibits microglial activation and suppresses the release of pro-inflammatory cytokines in Parkinson’s animal model induced by LPS [54, 55]. Furthermore, ginsenoside, the active substance of ginseng, attenuates microglial activation induced by LPS. The effect was related to inhibiting pro-inflammatory cytokine release in the hippocampal DG of male C57BL/6J mice [27]. A previous study reported that yokukansan, whose components are similar to NYT (Japanese angelica root, atractylodes rhizome, poria sclerotium, and glycyrrhiza), inhibits microglial activation in the hippocampal DG of schizophrenia animal models [56]. Thus, our results suggest that the active components of NYT may decrease the release of pro-inflammatory cytokine to attenuate microglial activation in the hippocampus.
Interestingly, the effect of NYT on microglia activation occurs only in the hippocampal DG region. Previous studies reported that NYT improved immature neurons of male C57BL/6 mice in the DG region [24], which DG had the most newborn cells compared to other areas of the hippocampus [57, 58]. On the other hand, previous studies reported that NYT or its components have a therapeutic effect on hippocampal DG [24, 27, 56]. However, to the best of our knowledge, there are no studies examining the effect of NYT simultaneously in all areas of the hippocampus than DG only. Thus, the mechanism is still unclear. Therefore, further studies are needed.
However, NYT did not affect GFAP expression in the hippocampus, suggesting that NYT did not inhibit astrocyte activation induced by LPS. This result coincides with the previous report that NYT treatments had no effect on astrocytes cell numbers in the hippocampus DG of male C57BL/6 mice induced by chronic administration of corticosterone [24].
On the other hand, we found that NYT did not significantly affect weight loss. Bodyweight is multi-complex regulated by the interaction of some processes [59]. Our results suggest that NYT has an effect on the hippocampus and depressive behavior, but not on weight loss recovery. Therefore, body weight may be regulated complex system including other brain areas than the hippocampus.
Although the current study indicates the effectiveness of NYT in improving depression conditions, we have noted some limitations. First, we found NYT improved depressive-like behavior and reduced microglial activation. However, the molecular mechanism of the causal relationship between behavioral abnormalities and glial pathological changes in the rat depression models induced by LPS was not checked. Second, we only performed one depressive-like behavior test and did not use other antidepressants as a control. In addition, we found a difference in body weight between the four groups in the present study. Body weight bias is associated with undesirable physical and behavioral test outcomes [48]. The sham and NYT groups had a significant difference in body weight than the LPS group, which might be a bias in their effect on behavior test results. At the same time, the LPS and NYT-LPS groups did not find a significant difference in body weight that the influence of weight bias on behavior tests may be ruled out. Further studies are warranted to check the molecular mechanism of how NYT improves depressive-like behavior by reducing neuroinflammation.
CONCLUSIONS
In conclusion, the present study shows that NYT alleviates depressive-like behavior. The effect may be related to the attenuation of glial cell activation in the hippocampus of LPS-induced depression animal models. These findings can be used as a basis to examine the role of glial pathological changes in the pathogenesis of depression.
Ethical Approval
All the experimental protocol was reviewed and approved by the Shimane University Animal Ethics Committee under the guidelines of the National Health and Medical Research Council of Japan (Authorization No: IZ2-104).
Author Contributions
Conceptualization, M.A.J., M.H., and M.I.; methodology, M.A.J., M.H., and M.I.; software, M.A.J., M.H., K.T., and M.I.; validation, M.A.J., M.H., K.T., S.J.F.J., R.M., S.M., M.N., K.O., S.H., R.W., T.M., A.J.T., J.H., and M.I.; formal analysis, M.A.J., M.H., K.T., and M.I.; investigation, M.A.J., M.H., K.T., and M.I.; resources, M.H., and M.I.; data curation, M.A.J., M.H., K.T., S.J.F.J., R.M., and M.I.; writing—original draft preparation, M.A.J., M.H., and M.I.; writing—review and edit-ing, S.M., M.N., K.O., S.H., R.W., T.M., A.J.T., and J.H.; visualization, M.A.J., M.H., and M.I.; supervision, M.H., and M.I.; project administration, M.H., and M.I.; funding acquisition, M.H., and M.I. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The author’s thankful to Tsumura Corporation (Tokyo, Japan) for its kindness in supplying ninjin’yoeito dry powder extract. We are particularly thankful to Ms. Sakura Hino, Ms. Satoko Yano, and Ms. Tomoko Araki (staff of the Department of Psychiatry, Faculty of Medicine, Shimane University) for their kind assistance.
Funding
This study was supported and funded by JSPS KAKENHI grant number 19K8046.
Conflict of interest
M.I. received lecture fees from Technomics, Fuji Keizai, Novartis, Yoshitomiyakuhin, Pfizer, MSD, Meiji Seika Pharma, Eisai, Otsuka, Sumitomo Dainippon Pharma, Mochida, Janssen, Takeda, and Eli Lilly.
REFERENCES
- 1) Katon W. The epidemiology of depression in medical care. Int J Psychiatry Med 1987;17:93-112. doi: 10.2190/XE8W-GLCJ-KEM6-39FH.
- 2) Raič M. Depression and heart diseases: leading health problems. Psychiatr Danub 2017;29 Suppl 4:770-7.
- 3) Malhi GS, Mann JJ. Depression. Lancet 2018;392:2299-312. doi: 10.1016/S0140-6736(18)31948-2.
- 4) Rakel RE. Depression. Prim Care 1999;26:211-24. doi: 10.1016/S0095-4543(08)70003-4.
- 5) Buoli M, Caldiroli A, Altamura AC. Psychotic versus non-psychotic major depressive disorder: A comparative naturalistic study. Asian J Psychiatr 2013;6:333-7. doi: 10.1016/j.ajp.2013.02.003.
- 6) Eby GA, Eby KL. Rapid recovery from major depression using magnesium treatment. Med Hypotheses 2006;67:362-70. doi: 10.1016/j.mehy.2006.01.047.
- 7) Belmaker RH, Agam G. Major depressive disorder. N Engl J Med 2008;358:55-68. doi: 10.1056/NEJMra073096.
- 8) Rajkowska G, Miguel-Hidalgo J. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets 2007;6:219-33. doi: 10.2174/187152707780619326.
- 9) Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry 2000;48:766-77. doi: 10.1016/S0006-3223(00)00950-1.
- 10) Czéh B, Nagy SA. Clinical findings documenting cellular and molecular abnormalities of glia in depressive disorders. Front Mol Neurosci 2018;11:56. doi: 10.3389/fnmol.2018.00056.
- 11) Stockmeier CA, Rajkowska G. Cellular abnormalities in depression: evidence from postmortem brain tissue. Dialogues Clin Neurosci 2004;6:185-97. doi: 10.31887/DCNS.2004.6.2/cstockmeier.
- 12) Banasr M, Chowdhury GMI, Terwilliger R, et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 2010;15:501-11. doi: 10.1038/mp.2008.106.
- 13) Arauchi R, Hashioka S, Tsuchie K, et al. Gunn rats with glial activation in the hippocampus show prolonged immobility time in the forced swimming test and tail suspension test. Brain Behav 2018;8:e01028. doi: 10.1002/brb3.1028.
- 14) Wang YL, Han QQ, Gong WQ, et al. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J Neuroinflammation 2018;15:21. doi: 10.1186/s12974-018-1054-3.
- 15) Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci 2015;38:637-58. doi: 10.1016/j.tins.2015.08.001.
- 16) Yamawaki Y, Yoshioka N, Nozaki K, et al. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res 2018;1680:13-38. doi: 10.1016/j.brainres.2017.12.004.
- 17) Arioz BI, Tastan B, Tarakcioglu E, et al. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front Immunol 2019;10:1511. doi: 10.3389/fimmu.2019.01511.
- 18) Remus JL, Dantzer R. Inflammation models of depression in rodents: relevance to psychotropic drug discovery. Int J Neuropsychopharmacol 2016;19:pyw028. doi: 10.1093/ijnp/pyw028.
- 19) Planchez B, Surget A, Belzung C. Animal models of major depression: drawbacks and challenges. J Neural Transm 2019;126:1383-408. doi: 10.1007/s00702-019-02084-y.
- 20) Miyano K, Nonaka M, Uzu M, Ohshima K, Uezono Y. Multifunctional actions of ninjinyoeito, a japanese kampo medicine: accumulated scientific evidence based on experiments with cells and animal models, and clinical studies. Front Nutr 2018;5:93. doi: 10.3389/fnut.2018.00093.
- 21) Takayama S, Arita R, Ohsawa M, et al. Perspectives on the use of ninjin’yoeito in modern medicine: a review of randomized controlled trials. Evidence-based Complement Alternat Med 2019;2019:9590260. doi: 10.1155/2019/9590260.
- 22) Kudoh C, Arita R, Honda M, et al. Effect of ninjin’yoeito, a kampo (traditional Japanese) medicine, on cognitive impairment and depression in patients with alzheimer’s disease: 2 years of observation. Psychogeriatrics 2016;16:85-92. doi: 10.1111/psyg.12125.
- 23) Ohsawa M, Tanaka Y, Ehara Y, Makita S, Onaka K. A possibility of simultaneous treatment with the multicomponent drug, ninjin’yoeito, for anorexia, apathy, and cognitive dysfunction in frail alzheimer’s disease patients: an open-label pilot study. J Alzheimer’s Dis Rep 2017;1:229-35. doi: 10.3233/adr-170026.
- 24) Murata K, Fujita N, Takahashi R, Inui A. Ninjinyoeito improves behavioral abnormalities and hippocampal neurogenesis in the corticosterone model of depression. Front Pharmacol 2018;9:1216. doi: 10.3389/fphar.2018.01216.
- 25) Cheong MH, Lee SR, Yoo HS, et al. Anti-inflammatory effects of polygala tenuifolia root through inhibition of NF-κB activation in lipopolysaccharide-induced BV2 microglial cells. J Ethnopharmacol 2011;137:1402-8. doi: 10.1016/j.jep.2011.08.008.
- 26) Kumar A, Rinwa P, Dhar H. Microglial inhibitory effect of ginseng ameliorates cognitive deficits and neuroinflammation following traumatic head injury in rats. Inflammopharmacology 2014;22:155-67. doi: 10.1007/s10787-013-0187-3.
- 27) Park SM, Choi MS, Sohn NW, Shin JW. Ginsenoside Rg3 attenuates microglia activation following systemic lipopolysaccharide treatment in mice. Biol Pharm Bull 2012;35:1546-52. doi: 10.1248/bpb.b12-00393.
- 28) Wang X, Li M, Cao Y, et al. Tenuigenin inhibits LPS-induced inflammatory responses in microglia via activating the Nrf2-mediated HO-1 signaling pathway. Eur J Pharmacol 2017;809:196-202. doi: 10.1016/j.ejphar.2017.05.004.
- 29) Castagné V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci 2011; Chapter 8: Unit 8.10A. doi: 10.1002/0471142301.ns0810as55.
- 30) Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. J Vis Exp 2011;59:e3638. doi: 10.3791/3638.
- 31) Yankelevitch-Yahav R, Franko M, Huly A, Doron R. The forced swim test as a model of depressive-like behavior. J Vis Exp 2015;97:e52587. doi: 10.3791/52587.
- 32) Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system’s immune response. Neurol Res 2005;27:685-91. doi: 10.1179/016164105X49463a.
- 33) Streit WJ, Mrak RE, Griffin WST. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 2004;1:14. doi: 10.1186/1742-2094-1-14.
- 34) Ye X, Zhu M, Che X, et al. Lipopolysaccharide induces neuroinflammation in microglia by activating the MTOR pathway and downregulating Vps34 to inhibit autophagosome formation. J Neuroinflammation 2020;17:18. doi: 10.1186/s12974-019-1644-8.
- 35) Greenhalgh AD, David S, Bennett FC. Immune cell regulation of glia during CNS injury and disease. Nat Rev Neurosci 2020;21:139-52. doi: 10.1038/s41583-020-0263-9.
- 36) Chen Y, Qin C, Huang J, et al. The role of astrocytes in oxidative stress of central nervous system: a mixed blessing. Cell Prolif 2020;53:e12781. doi: 10.1111/cpr.12781.
- 37) Song QH, Toriizuka K, Iijima K, Watanabe K, Cyong JC. Effects of ninjin-yoei-to (Rensheng-yangrong-tang), a Kampo medicine, on brain monoamine and nerve growth factor contents in mice with olfactory bulb lesions. J Tradit Med 2001;18:64-70.
- 38) Russo R, Autore G, Severino L. Pharmaco-toxicological aspects of herbal drugs used in domestic animals. Nat Prod Commun 2009;4:1777-84.
- 39) Shimada Y. Adverse effects of kampo medicines. Intern Med 2021;61:29-35. doi: 10.2169/internalmedicine.6292-20.
- 40) Elgarf ASA, Aboul-Fotouh S, Abd-Alkhalek HA, et al. Lipopolysaccharide repeated challenge followed by chronic mild stress protocol introduces a combined model of depression in rats: reversibility by imipramine and pentoxifylline. Pharmacol Biochem Behav 2014;126:152-62. doi: 10.1016/j.pbb.2014.09.014.
- 41) Guo Y, Cai H, Chen L, et al. Quantitative profiling of neurotransmitter abnormalities in the hippocampus of rats treated with lipopolysaccharide: focusing on kynurenine pathway and implications for depression. J Neuroimmunol 2016;295-296:41-6. doi: 10.1016/j.jneuroim.2016.04.006.
- 42) Jiang P, Guo Y, Dang R, et al. Salvianolic acid B protects against lipopolysaccharide-induced behavioral deficits and neuroinflammatory response: involvement of autophagy and NLRP3 inflammasome. J Neuroinflammation 2017;14:239. doi: 10.1186/s12974-017-1013-4.
- 43) Azis IA, Hashioka S, Tsuchie K, et al. Electroconvulsive shock restores the decreased coverage of brain blood vessels by astrocytic endfeet and ameliorates depressive-like behavior. J Affect Disord 2019;257:331-9. doi: 10.1016/j.jad.2019.07.008.
- 44) Hovens I, Nyakas C, Schoemaker R. A novel method for evaluating microglial activation using ionized calcium-binding adaptor protein-1 staining: cell body to cell size ratio. Neuroimmunol Neuroinflammation 2014;1:82-8.
- 45) Zhang S, Wu M, Peng C, Zhao G, Gu R. GFAP expression in injured astrocytes in rats. Exp Ther Med 2017;14:1905-8. doi: 10.3892/etm.2017.4760.
- 46) Liaury K, Miyaoka T, Tsumori T, et al. Minocycline improves recognition memory and attenuates microglial activation in Gunn rat: a possible hyperbilirubinemia-induced animal model of schizophrenia. Prog Neuro-Psychopharmacology Biol Psychiatry 2014;50:184-90. doi: 10.1016/j.pnpbp.2013.12.017.
- 47) Limoa E, Hashioka S, Miyaoka T, et al. Electroconvulsive shock attenuated microgliosis and astrogliosis in the hippocampus and ameliorated schizophrenia-like behavior of Gunn rat. J Neuroinflammation 2016;13:230. doi: 10.1186/s12974-016-0688-2.
- 48) Lee S, Nam H, Kim J, et al. Body weight changes of laboratory animals during transportation. Asian-Australasian J Anim Sci 2012;25:286-90. doi: 10.5713/ajas.2011.11227.
- 49) Watanabe K, Matsuura K, Gao P, et al. Traditional japanese kampo medicine: clinical research between modernity and traditional medicine: the state of research and methodological suggestions for the future. Evidence-Based Complement Altern Med 2011;2011:513842. doi: 10.1093/ecam/neq067.
- 50) Sato S, Tamura M, Takahashi T, Arai T. Ninjin’yoeito (tsumura) extract; a kampo (traditional herbal medicine) improved apathy in a mild cognitive impairment patient. Clin Neuropsychopharmacol Ther 2018;9:17-9. doi: 10.5234/cnpt.9.17.
- 51) Matsumoto T, Takiyama M, Sakamoto T, et al. Pharmacokinetic study of ninjin’yoeito: absorption and brain distribution of ninjin’yoeito ingredients in mice. J Ethnopharmacol 2021;279:114332. doi: 10.1016/j.jep.2021.114332.
- 52) Watanabe N, Iimura K, Hotta H. Influence of intragastric administration of traditional Japanese medicine, ninjin’yoeito, on cerebral blood flow via muscarinic acetylcholine receptors. Evidence-Based Complement Altern Med 2021;2021:9930023. doi: 10.1155/2021/9930023.
- 53) Araki R, Hiraki Y, Nishida S, Inatomi Y, Yabe T. Gomisin N ameliorates lipopolysaccharide-induced depressive-like behaviors by attenuating inflammation in the hypothalamic paraventricular nucleus and central nucleus of the amygdala in mice. J Pharmacol Sci 2016;132:138-44. doi: 10.1016/j.jphs.2016.09.004.
- 54) Yuan HL, Li B, Xu J, et al. Tenuigenin protects dopaminergic neurons from inflammation-mediated damage induced by the lipopolysaccharide. CNS Neurosci Ther 2012;18:584-90. doi: 10.1111/j.1755-5949.2012.00347.x.
- 55) Fan Z, Liang Z, Yang H, Pan Y, Zheng Y, Wang X. Tenuigenin protects dopaminergic neurons from inflammation via suppressing NLRP3 inflammasome activation in microglia. J Neuroinflammation 2017;14:256. doi: 10.1186/s12974-017-1036-x.
- 56) Furuya M, Miyaoka T, Tsumori T, et al. Yokukansan promotes hippocampal neurogenesis associated with the suppression of activated microglia in Gunn rat. J Neuroinflammation 2013;10:145. doi: 10.1186/1742-2094-10-145.
- 57) Sahay A, Drew MR, Hen R. Dentate gyrus neurogenesis and depression. Prog Brain Res 2007;163:697-722. doi: 10.1016/S0079-6123(07)63038-6.
- 58) Umschweif G, Greengard P, Sagi Y. The dentate gyrus in depression. Eur J Neurosci 2021;53:39-64. doi: 10.1111/ejn.14640.
- 59) Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes 2015;39:1188-96. doi: 10.1038/ijo.2015.59.