2022 Volume 39 Issue 1 Pages 27-32
2022 Volume 39 Issue 1 Pages 27-32
Objectives: The aim of this study was to evaluate the prognostic impact of echocardiographic indices in our real-world patients with symptomatic severe aortic stenosis (AS) patients who underwent transcatheter aortic valve implantation (TAVI). Methods: We retrospectively reviewed the data of 54 patients with severe AS who underwent TAVI between September 2018 and May 2020. The presence and extent of cardiac damage was evaluated on baseline transthoracic echocardiography. Results: The mean age of the cohort was 87 ± 4 years with 19 (35%) men included. With regard to cardiac damage, 3 patients (5.5%) were classified under Stage 1 (LV damage), 41 (75.9%) under Stage 2 (mitral valve or LA damage), 9 (16.6%) under Stage 3 (tricuspid or pulmonary artery vasculature damage), and 1 (1.9%) under Stage 4 (RV damage). The cumulative all cause and cardiovascular mortalities were 5.5% (n = 3) and 0%, respectively. Three patients experienced valve-related events (VRE, hospitalization for congestive heart failure) within the follow-up period, of whom 1 patient each was categorized under stages 2, 3, and 4. No significant relationship between VRE and the stage of cardiac damage was found. The relative wall thickness (RWT) of patients with VRE was significantly greater than those without VRE (0.71 ± 0.05 vs. 0.60 ± 0.08, P < 0.016). A RWT cut-off value of 0.66 (sensitivity, 100%; specificity of 72%) was obtained to detect the presence of VRE. Conclusions: Patients with smaller LV size and concentric hypertrophy are at high risk for heart failure hospitalization after TAVI.
Symptomatic severe aortic stenosis (AS) has a dismal prognosis. Thus, early intervention is strongly recommended in all patients. Intervention is recommended for patients diagnosed with symptomatic severe AS, unless with contraindications to the intervention or with a predicted survival <1 year [1]. Surgical aortic valve replacement (SAVR) for severe AS has been the gold standard treatment and the first choice for younger patients with lower surgical risk, conferring a positive long-term prognosis [2, 3]. However, with the clinical application of transcatheter aortic valve implantation (TAVI) since 2002, it has become necessary to decide whether SAVR or TAVI should be selected. Although TAVI was initially limited to high-risk cases among the elder patients, its use has increased as the technology has advanced and the procedure was further improved [4]. The selection of SAVR or TAVI should be done after considering the patient’s age, anatomical characteristics, comorbidities, and frailty, as well as the durability of the valve replacement [5]. The present guidelines, jointly released by Japanese Circulation Society (JCS), Japanese Society for Cardiovascular Surgery (JCSC), Japanese Association for Thoracic Surgery (JATS), and Japanese Society for Vascular Surgery (JSVS) in 2020, offer an index of prioritization that suggests using TAVI in patients aged ≥80 years and SAVR in those aged <75 years [1].
Recently, a new staging system for severe AS has been proposed to quantify the extent of cardiac anatomical and functional damage, as evaluated by echocardiography in patients with AS [6]. Vollema et al. demonstrated that the stage of cardiac damage was independently associated with all-cause mortality and the combined endpoint of all-cause mortality, stroke, and cardiac related hospitalization in patients with symptomatic severe AS patients [7]. The aim of this study was to evaluate the prognostic impact of echocardiographic indices in our real-world patients with symptomatic severe AS patients who underwent TAVI.
Patients
In this study, we retrospectively reviewed the data of 54 patients with severe AS who underwent TAVI between September 2018 and May 2020. Severe AS was defined in concordance with the JCS/JSCS/JATS/JSVS 2020 guidelines as a peak aortic jet velocity ≥4.0 m/s and/or a mean aortic valve (AV) gradient ≥40 mmHg and/or an aortic valve area <1.0 cm2 [1]. In total, the data of 54 patients were analyzed. The study protocol was approved by the ethics committee of the Shimane University Faculty of Medicine in Izumo, Japan.
Echocardiographic data
A standard echocardiographic examination that included comprehensive two-dimensional (2D) and Doppler echocardiography using a multi-window approach was performed by experienced sonographers prior to the TAVI procedure in all patients [8]. The severity of AS was assessed by the peak AV velocity, mean AV pressure gradient, and AV area, which was calculated using the continuity equation with the left ventricular (LV) outflow tract diameter and flow velocity. LV dimensions were measured from the parasternal long axis view at end-diastole (LVDd) and at end-systole (LVDs). The end-diastolic volume (EDV) and end-systolic volume (ESV) of the LV were measured using Simpson’s method on 2D images from the apical four- and two-chamber views. LV ejection fraction (LVEF) was calculated using the equation 100 × (EDV –ESV) / EDV. LV mass was estimated using diastolic measurements of the LV internal diameter and the wall thickness from the parasternal long axis view: LV mass (g) = 0.8 × (1.04 × [(intraventricular septum thickness + LV internal dimension + posterior wall thickness)3 – (LV internal dimension)3]) + 6 g. Afterward, LV mass index (LVMI) was calculated as LV mass/body surface area (BSA). LV hypertrophy was defined as ≥115 mL/BSA for men and ≥95 mL/BSA for women. Calculation of relative wall thickness (RWT) with the formula (2 × posterior wall thickness) /(LV internal diameter at end-diastole) permits categorization of an increase in LV mass as either concentric (RWT >0.42) or eccentric (RWT ≤0.42) hypertrophy; defined by a normal LV mass with increased RWT. The anteroposterior left atrial (LA) dimension (LAD) was measured in the parasternal long-axis view. 2D measurements were used to calculate the LA volume (LAV) at the end-systole (just prior to the opening of the mitral valve) using Simpson’s method in the apical four- and two-chamber views. LAV index was calculated per BSA. The transmitral flow velocity was recorded from an apical four-chamber view by placing the sample volume at the level of the mitral valve leaflet tips. To assess the diastolic function, mitral inflow velocities during early (E) and late (A) diastolic filling were obtained and the E/A ratio was calculated. Early diastolic velocity (e’) was obtained from the septal annulus motion of the LV on tissue Doppler imaging. The ratio between the peak E and e’ was calculated (E/e’). RV systolic function was evaluated using the fractional area change (the percentage of change in the RV cavity area between end-diastole and end-systole from the apical four-chamber view) and tricuspid annular plane systolic excursion (TAPSE). The transtricuspid pressure gradient (TRPG) was estimated by continuous-wave Doppler using the simplified Bernoulli equation of tricuspid regurgitation peak velocity.
Diagnostic staging classification
The presence and extent of cardiac damage was evaluated on baseline transthoracic echocardiography [6]. Stages of cardiac damage were defined as followings: Stage 0, no signs of cardiac damage; Stage 1, LV damage (LVEF <50%, LVMI >95 g/m2 for women or >115 g/m2 for men, or E/e’ >14); Stage 2, mitral valve or LA damage (LA volume index >34 mL/m2 or mitral regurgitation grade ≥3 or presence of atrial fibrillation); Stage 3, tricuspid valve or pulmonary artery vasculature damage (systolic pulmonary artery pressure ≥60 mmHg or tricuspid regurgitation grade ≥3); and Stage 4, RV damage (TAPSE <16 mm).
Follow-up
All patients were followed up after TAVI. Follow-up data were obtained from a detailed review of all medical records. Adverse valve-related events (VRE) were defined as cardiac death or hospitalization for congestive heart failure (HF). Cardiac death was defined as sudden death, death from HF, or myocardial infarction.
Statistical analysis
Categorical variables were reported as numbers and percentages, while quantitative variables were described as a mean ± standard deviation. The distribution of quantitative variables was evaluated using the Shapiro-Wilk test. Normally distributed groups of quantitative variables were compared using t-tests and non-normally distributed groups of continuous variables were compared using the Mann-Whitney U test. The chi-square test was used to compare samples of qualitative variables. Statistically significance was set at P < 0.05. A receiver operating characteristics (ROC) curve was generated and the area under the curve was calculated to determine the optical cut-off value for predicting the VRE. Survival analysis was performed using a Kaplan-Meier analysis and differences between groups were calculated with the log-rank test. All statistical analyses were performed using SPSS Statistics Desktop Version 22.0 (IBM, Armonk, NY).
Baseline characteristics
The baseline characteristics of the patients are depicted in Table 1. The mean age of the cohort was 87 ± 4 years with 19 (35%) men included. The mean aortic peak velocity, mean pressure gradient, and mean AV area were 4.9 ± 0.8 m/s, 57 ± 21 mmHg, and 0.62 ± 0.15 cm2, respectively. The mean follow-up duration was 431 days. With regard to cardiac damage, 3 patients (5.5%) were classified under Stage 1 (LV damage), 41 (75.9%) under Stage 2 (mitral valve or LA damage), 9 (16.6%) under Stage 3 (tricuspid or pulmonary artery vasculature damage), and 1 (1.9%) under Stage 4 (RV damage).
|
All patients N = 54 |
VRE (+) N = 3 |
VRE (-) N = 51 |
P value | |
|---|---|---|---|---|
| Age (years) | 87 ± 4 | 89 ± 1 | 87 ± 4 | 0.271 |
| Male | 19 (35%) | 1 (33%) | 18 (35%) | 0.945 |
| BSA (m2) | 1.40 ± 0.16 | 1.35 ± 0.10 | 1.41 ± 0.17 | 0.686 |
| AV peak velocity (m/s) | 4.9 ± 0.8 | 4.2 ± 0.7 | 4.9 ± 0.8 | 0.138 |
| Mean AV PG (mmHg) | 57 ± 21 | 42 ± 12 | 58 ± 21 | 0.138 |
| AV area (cm2) | 0.62 ± 0.15 | 0.71 ± 0.09 | 0.62 ± 0.16 | 0.343 |
| LVDd (mm) | 41 ± 5 | 34 ± 2 | 41 ± 5 | 0.005 |
| LVDs (mm) | 27 ± 5 | 20 ± 1 | 27 ± 5 | 0.002 |
| LVMI (g/m2) | 190 ± 78 | 113 ± 17 | 194 ± 83 | 0.002 |
| RWT | 0.61 ± 0,08 | 0.71 ± 0.05 | 0.60 ± 0.08 | 0.016 |
| LVEF (%) | 63 ± 14 | 71 ± 5 | 63 ± 12 | 0.127 |
| LAD (mm) | 42 ± 6 | 37 ± 4 | 42 ± 6 | 0.172 |
| LAVI (mL/m2) | 58.7 ± 20.8 | 45.7 ± 8.8 | 59.5 ± 21.1 | 0.125 |
| E/A | 0.8 ± 0.4 | 0.5 ± 0.2 | 0.8 ± 0.4 | 0.251 |
| E/e’ | 24.8 ± 11.3 | 32.6 ± 20.7 | 24.3 ± 10.7 | 0.565 |
| TR PG (mmHg) | 27 ± 9 | 28 ± 7 | 27 ± 9 | 0.727 |
| RV FAC (%) | 45 ± 8 | 48 ± 5 | 45 ± 9 | 0.656 |
| TAPSE (mm) | 20.9 ± 2.6 | 20.6 ± 4.2 | 20.9 ± 2.6 | 0.912 |
VRE: valve-related events; AV: aortic valve; PG: pressure gradient; LVDd: left ventricular (LV) dimension at end-diastole; LVDs: LV dimension at end-systole; LVMI: LV mass index; RWT: relative wall thickness; LVEF: LV ejection fraction; LAD: left atrial (LA) dimension; LAVI: LA volume index; TR: tricuspid regurgitation; RV: right ventricle; FAC: fractional area change; TAPSE: tricuspid annular plane systolic excursion
Outcomes
The cumulative 1-year all cause and cardiovascular mortalities were 5.5% (n = 3) and 0%, respectively. Three patients experienced VRE (hospitalization for congestive HF) within the follow-up period, of whom 1 patient each was categorized under stages 2, 3, and 4. No significant relationship between VRE and the stage of cardiac damage was found. The patients with VRE had significantly smaller LV size (LVDd, 34 ± 2 mm vs. 41 ± 5 mm, P < 0.005; LVDs, 20 ± 1 mm vs. 27 ± 5 mm, P < 0.002) and LVMI (113 ± 17 vs. 194 ± 83, P < 0.002) than patients with no VRE (Table 1). The RWT of patients with VRE was significantly greater than those without VRE (0.71 ± 0.05 vs. 0.60 ± 0.08, P < 0.016). A RWT cut-off value of 0.66 (sensitivity, 100%; specificity of 72%) was used to detect the presence of VRE. The area under the curve was 0.66 (P = 0.022). In patients with RWT ≥0.66, VRE free-survival rate during follow-up was 75 ± 13%. In contrast, VRE free-survival rate during follow-up was 100% in patients with RWT <0.66 (Figure 1).
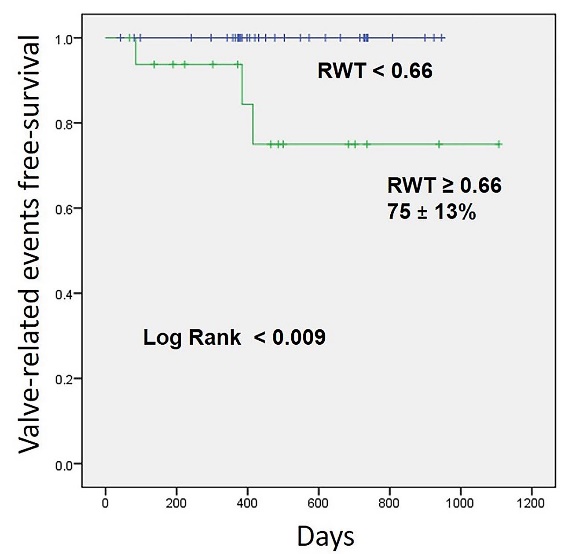
Kaplan-Meier curves showing valve-related events free-survival in patients with relative wall thickness (RWT) <0.66 and ≥0.66.
A previous study has demonstrated that the staging system of cardiac damage provided accurate prognostic value in patients undergoing TAVI. Stages 2-4 conferred an increased risk of all-cause mortality and cardiovascular mortality, compared with stages 0-1 at 1 year post TAVI [9]. In our cohort that was composed of older patients, there were no significant relationships between VRE and the stage of cardiac damage. The patients who were hospitalized for congestive HF within the follow-up period had a significantly smaller LV size and greater RWT (i.e., concentric hypertrophy).
Maintenance of cardiac output in patients with severe AS imposes a chronic increase in LV pressure. In response, the LV typically undergoes hypertrophic remodeling characterized by myocyte hypertrophy and increased wall thickness. LV remodeling may manifest as concentric remodeling, concentric hypertrophy, or eccentric hypertrophy. Based on LaPlace’s law, LV remodeling reduces wall stress; thus, it is considered one of the important compensatory mechanisms in maintaining LV ejection performance. However, in patients with severe AS, several studies have demonstrated that increased LV hypertrophic remodeling is associated with more severe LV dysfunction and HF symptoms, as well as higher mortality [10, 11]. Cardiac hypertrophy in response to pressure overload involves both adaptive and maladaptive processes [12]. While LV hypertrophic remodeling may reduce wall stress, it may have long-term deleterious effects that translate into impaired LV performance and worse clinical outcomes. A small LV radius with increased relative wall thickness allows for stress normalization. To maintain stroke volume and ejection performance, however, the presence of increased filling pressure and oxygen consumption is linked. Thus, there are diastolic dysfunction and impaired coronary blood flow reserve in patients with smaller LV size and larger RWT. Petrov et al. demonstrated that the wide variation in the LV adaptation to the pressure overload of AS [13]. Shishido et al. found a significant regression in LVMI between the time prior to and 1 year after TAVI [14]. Previous studies indicated that patients with less fibrosis exhibited adaptive regression of LVH after SAVR [13, 15]. The LV hypertrophic response is progressively followed by enlargement of the interstitial space with reactive fibrosis and, at a later stage, with replacement fibrosis and cell death. Assessment of LV fibrosis may predict adaptive regression of LVH after TAVI [16].
In our cohort, 1 patient was classified with Stage 4 damage and was hospitalized due to HF after TAVI. Asami et al. have demonstrated that RV dysfunction at baseline was associated with a more than two-fold increased risk of cardiovascular death at 1 year after TAVI [17]. In patients with severe AS, long-standing elevated left-sided filling pressures can lead to pulmonary vascular remodeling and pulmonary arterial hypertension, and result in compensatory RV remodeling, dilatation, and eventual RV impairment [18]. The recognition of advanced stages of cardiac damage may improve risk assessment of patients undergoing TAVI and modulate subsequent follow-up and management strategies.
Our study had several limitations. First, it was a retrospective study with a relatively small sample size and small event rates. Second, although follow-up event data were collected, frailty and quality of life were not assessed. Finally, the study was not designed to assess and compare the event rates among patients who had surgical interventions. Thus, in order to verify the present results, further prospective large-scale studies are needed.
Patients with smaller LV size and concentric hypertrophy are at high risk for HF hospitalization after TAVI.
Conflict of interest
The authors declare no conflicts of interest associated with this manuscript.