2015 Volume 43 Issue 2 Pages 107-109
2015 Volume 43 Issue 2 Pages 107-109
In early 2014, dengue cases were reported from northern Mozambique, 30 years after the last outbreak. We identified potential dengue vector species in three northern towns, Pemba, Nampula and Nacala, and one southern town, Maputo, during the outbreak in April 2014. A major dengue vector species, Aedes (Stegomyia) aegypti, was found in all these towns. The dominant vector subspecies in the northern towns was Aedes aegypti aegypti, while Ae. aegypti formosus was dominant in Maputo. Considering the high proportion of Ae. aegypti aegypti and its high vector competence, the findings from this study suggest that Ae. aegypti aegypti was responsible for the outbreak in northern Mozambique.
Vector identification is crucial for controlling dengue. Vector control is the only way to reduce transmission, but an effective dengue vaccine has not yet been developed for practical use, and vector competence and ecology vary among vector taxa and populations.
Mattingly [1] reported that Aedes (Stegomyia) aegypti aegypti (Aaa) is predominantly anthropophilic and adapted to the human environment, while its subspecies, Ae. aegypti formosus (Aaf), is more associated with a forest environment. Initially, these subspecies were proposed based on the body color of a vast number of specimens collected from various areas of the world as well as their specific behavior observed in coastal Kenya. Adults of Aaa prefer an indoor environments and use artificial water containers for oviposition, while Aaf prefer an outdoor environment and the forest edge and breed in natural containers such as tree holes, rock pools and plant axils. Later, genetic differences were observed between Aaa and Aaf, even though they were collected from the same habitat within a certain area (Rabai) of coastal Kenya [2]. However, Brown et al. [3] demonstrated that Aaa and Aaf from other parts of Africa did not show any significant genetic differentiation. Moreover, African Ae. aegypti was clearly different from the populations collected from other continents. Thus, these subspecies require systematical reexamination to determine their taxonomic status.
Regarding vector competence, Aaa is highly susceptible to dengue and yellow fever virus, and is considered to be a more efficient virus vector than Aaf [4, 5]. However, this notion is based on the comparison between African Aaf and non-African Aaa. A recent study in Senegal revealed that DENV-2 susceptibility differs significantly among geographical populations within the country and that the extent of susceptibility was related to the subspecies composition of the population [5]. In Senegal, the relative abundance of Aaa increases from inland to coastline [5], which implies a higher risk of dengue outbreak in the coastal area.
This is also likely to be true for East Africa, because past outbreaks of dengue fever in east African countries such as Kenya, Tanzania, and Mozambique invariably occurred in coastal areas. Thus, we hypothesized that Aaa, having a high susceptibility to DENV, is predominant in these areas. Furthermore, the introduction of another major dengue vector species, Ae. albopictus, to East Africa is suspected because this species was recently found on the Eparse Islands in the Mozambique Channel [6]. In early 2014, several dengue cases were reported from the northern towns, Pemba and Nampula, Mozambique, 30 years after the last outbreak [7]. We identified the potential dengue vector species during the outbreak.
In Pemba and Nampula, we examined used tires for Ae. aegypti larvae using a dip net (5 × 7 cm, 500 μm mesh) in April 2014, because used tires are one of the major breeding habitats of dengue vectors and are easily accessible sources in dengue endemic areas of Southeast Asia [8]. Sampled larvae were fed with biscuits ad libitum and reared to adults in the laboratory. Within a day after emergence, the adult mosquitoes were killed with ethyl acetate and separated by sex. We pinned the adult mosquitoes carefully and examined the abdominal scales of female adults microscopically for subspecies identification by the presence of at least one white scale on the first tergum, following Huang’s key [9].
We examined used tires for potential dengue vectors in Nacala and Maputo during the same period (Fig. 1). Nacala is also on the northern coast of Mozambique, and there were fears that the disease would spread further into the town. The collection sites were spaced at least 200 m apart in Nacala and covered most of the town’s geographical area. Maputo is located in southern Mozambique, where dengue transmission has not been reported previously.
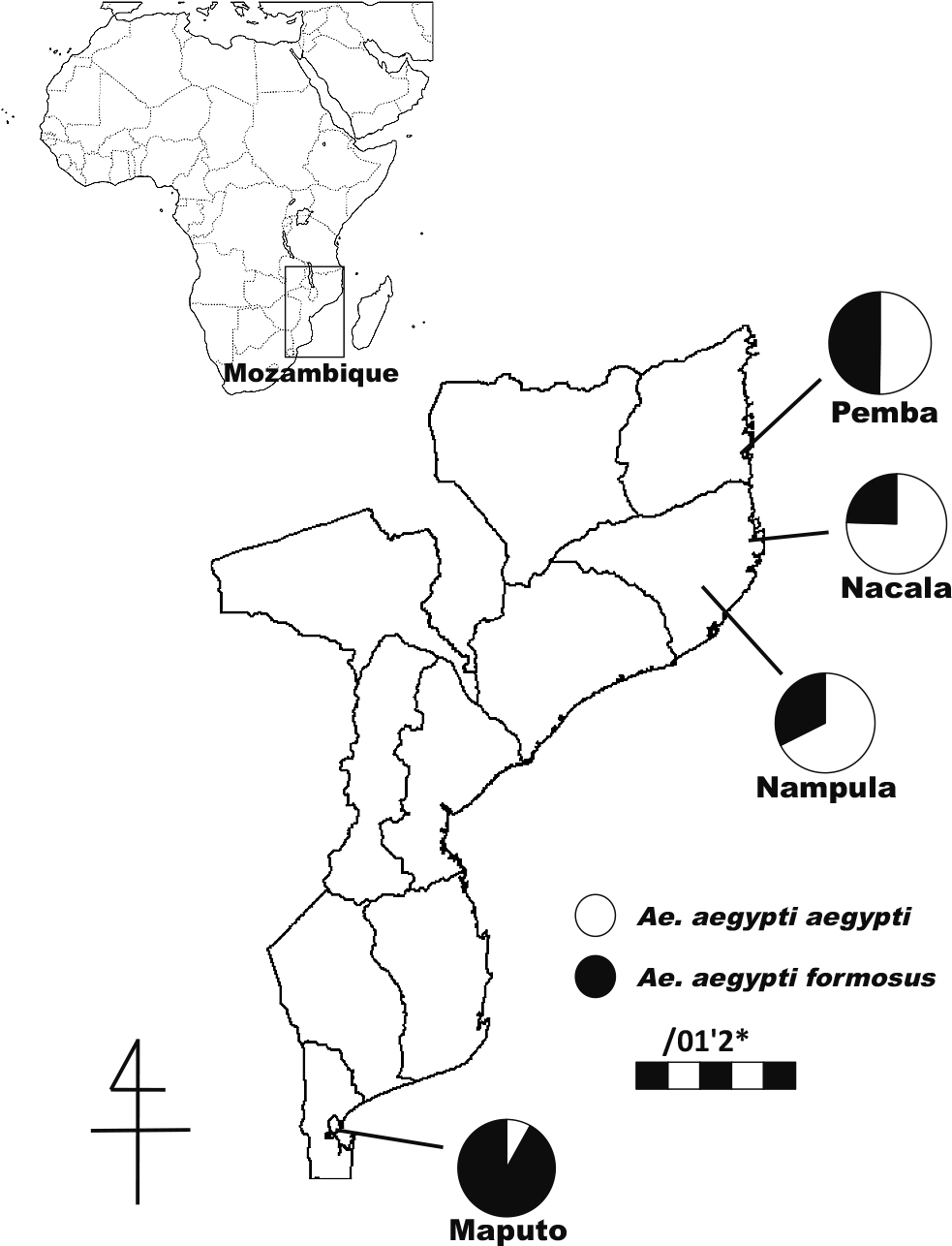
Sampling sites and subspecies compositions of Aedes aegypti aegypti (Aaa) and Ae. aegypti formosus (Aaf) in Mozambique.
In Pemba, 82 used tires were examined for Aedes larvae at 10 sites using a dip net. The collection sites were spaced at least 200 m apart to avoid biased collection from family population and covered most of the town’s geographical area. As a result, 25 (30.5%) of the used tires had water, and Ae. aegypti larvae were found in 18 of them (72.0% of the tires with water). Of 265 emerged adults, 133 (50.2%) were Aaa while the remainder (49.8%) were Aaf (Fig. 1). Aaa and Aaf were the only Aedes mosquito species found in this town.
Aaa was also abundant in Nampula and Nacala. We examined 72 tires and 61 tires at 11 sites and 10 sites in Nampula and Nacala, respectively. The collection sites were also spaced at least 200 m apart and covered most of the geographical area of the two towns. In total, 39 (54.2%) and 31 (50.8%) of the tires had water; Ae. aegypti larvae were found in 23 (59.0% of tires with water) tires and 20 (64.5% of tires with water) tires in Nampula and Nacala, respectively. The proportion of Aaa was 67.6% (n = 108) and 75.4% (n = 114) in Nampula and Nacala, respectively. Aaa and Aaf were the only Aedes mosquito species found in these towns.
In Maputo, 125 used tires at 11 sites were examined, and 83 (66.4%) of them contained water. Aedes aegypti larvae were sampled from 16 (19.3% of tires with water) tires, and the proportion of Aaa was 8.1% (n = 136). The collection sites were also spaced at least 200 m apart, and the survey area was limited to the western part of the city. Aaa and Aaf were the only Aedes species found in this area.
Considering the higher proportion of Aaa in northern Mozambique compared to Maputo in the south and its higher vector competence, the findings from this study suggest that Aaa was responsible for the outbreak in northern Mozambique. However, the number of collection sites was not enough to extrapolate the notion to other parts of Africa. Mosquito populations from several areas including inland regions need to be examined to test the hypothesis further.
In all collection sites, used tires were the main breeding habitats for Ae. aegypti. Because the environment of the four areas was consistent, we expected the habitats of Ae. aegypti to be similar between the northern and southern sites. Although Aaa and Aaf may prefer a different microhabitat for breeding as reported previously [1], we consider our data to be comparable among the collection sites, because mosquitoes were sampled from the same type of habitat. The different subspecies composition between northern and southern Mozambique would not have been affected greatly, because all of the specimens were collected from used tires.
This study also confirmed that Aaf breeds in human-generated water sources, despite past documentation of its association with a forest environment [1]. In fact, nearly all Aedes mosquitoes (91.9%) found in Maputo were Aaf, and both subspecies were sympatric in all collection sites. They are also sympatric at other locations along the East African coast and West Africa [2, 5]. The factor affecting the difference in subspecies composition between northern and southern Mozambique is unclear due to a lack of information from other parts of Mozambique. However, Aaa and Aaf may adapt to climate differently. In Senegal, two forms show a northwest–southeast cline in abundance. Only Aaa occurs in the north-western region, but it does not occur in the south-eastern region. The two subspecies are sympatric in the intermediate zone [5]. The south-eastern region is wetter, and its land cover is characterized by forest.
Dengue outbreaks in Africa are still sporadic, which may be partially attributable to the low susceptibility of Aaf to dengue 2 virus [4], although it is likely that many dengue cases go unrecognized and unreported in Africa. Studies in Senegal revealed that Ae. aegypti shows a generally low susceptibility to dengue 2 virus but that the susceptibility varied among populations within the country and depended on infected virus strains [5, 10, 11]. Interestingly, dengue outbreaks in Africa were mostly limited to coastal towns and islands, a phenomenon apparently due to virus introductions from outside Africa [12]. Our findings also imply the introduction of Aaa from outside Africa to the coastal towns [1], because this highly efficient vector widely inhabits tropical and subtropical areas outside Africa. An alternative hypothesis is that Aaa was domesticated within Africa [3]. In either case, the presence of a large number of domestic Ae. aegypti mosquitoes in northern Mozambique is a concern because of the rapid urbanization of Mozambique in recent years.
We are grateful to the staff at Direcção Provincial de Saúde de Cabo Delgado and Direcção Provincial de Saúde de Nampula and their local entomology teams for their help in collecting samples. We are also grateful to Dr. Nelson Cuamba and Dr. Francisco Mbofana for their support. This study was supported by the Program of Japan Initiative for Global Research Network on Infectious Diseases (J-GRID), MEXT, Japan. We have no conflicts of interest to declare.