2020 Volume 2 Issue 1 Pages 24-29
2020 Volume 2 Issue 1 Pages 24-29
Osteoporosis is associated with low bone density and pathological fractures induced by an imbalance in bone remodeling. It the most common bone disease and a social problem of an aging society. Norzoanthamine (NZ), a newly discovered marine alkaloid, has a suppressive effect on bone loss in osteoporosis animal models and is considered a candidate for anti-osteoporosis drug development. Truncated-norzoanthamine (TZ) is artificially synthesized, and possesses a structure similar to that of NZ. TZ and NZ have been shown to possess collagen protective activity. Here, we hypothesized that TZ also inhibits the bone loss observed in osteoporosis. We explored the effects of TZ treatment in an ovariectomized (OVX) osteoporosis mouse model. We observed that TZ suppressed the decrease in bone volume fraction, trabecular number, and bone mineral density in the femur of OVX mice as analyzed using micro-computed tomography, indicating an improvement in the bone mechanical characters. These findings indicate that TZ suppresses bone loss in OVX mice and suggest that TZ may offer therapeutic effects as a new anti-osteoporosis drug.
Bone remodeling is a dynamic process between bone formation by osteoblasts and bone resorption by osteoclasts. Normal bone remodeling provides bone the mature structure and maintains the normal calcium concentration in the body. Osteoporosis occurs due to imbalanced bone remodeling, with lower bone formation than bone resorption. In osteoporosis patients, the frequency of femur and vertebrae fractures are increased and could strongly limit the patient’s quality of life [1, 2]. With an increasingly aging population, osteoporosis has become a tremendous social problem. Furthermore, drug treatment for osteoporosis has not been satisfactorily established due to the diversity of this disease. Therefore, it is important to research and evaluate novel candidates as osteoporosis drugs.
The new marine alkaloid, norzoanthamine (NZ), was discovered in 1995 and is expected as a new drug for osteoporosis as it can suppress bone loss in ovariectomized mice, used as an osteoporosis model [3]. However, clinical use was challenging owing to the scarce availability from natural sources. In 2004, the total synthesis of NZ was reported; however, no reports regarding the effects of artificially synthesized NZ are available. [4] With regard to the mechanism of NZ, it has been reported that NZ demonstrates collagen protective activities in the extracellular matrix [5]. Based on this new activity, NZ could be developed as a new osteoporosis drug distinct from currently available ones (i.e., bisphosphonate). Recently, truncated norzoanthamine (TZ) (Fig. 1) has been developed as an easily synthesized substance, which includes two-thirds of the original NZ structure. Reportedly, TZ has collagen protective activities similar to NZ [6]. However, in vivo studies evaluating TZ are currently unavailable. Hence, to utilize TZ as a novel anti-osteoporosis drug in the future, the effect of TZ on bones needs to be investigated. Thus, the purpose of our research was to examine whether TZ demonstrates a suppressive effect on bone loss in an osteoporosis animal model induced by estrogen deficiency.
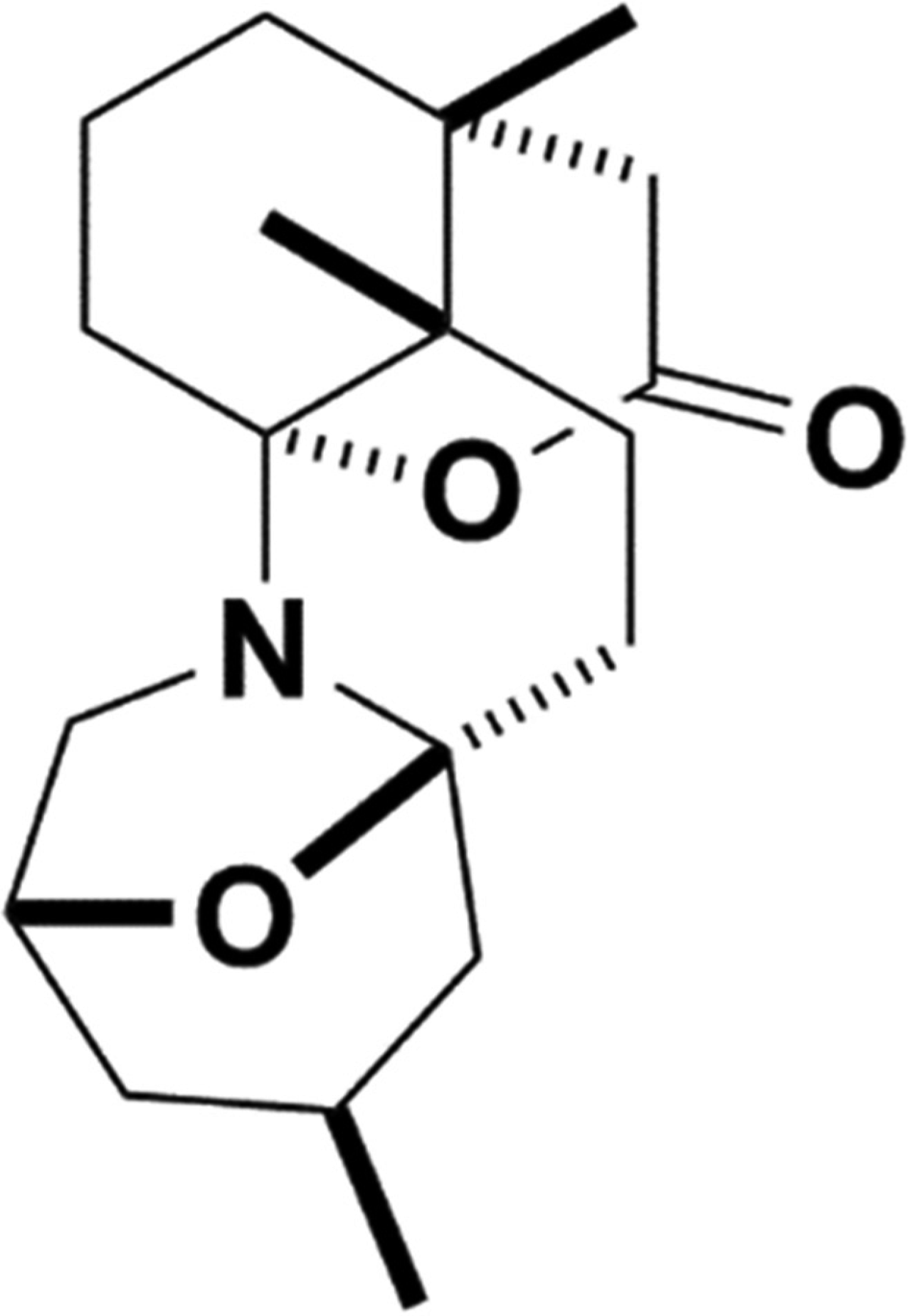
Chemical structure of truncated norzoanthamine (TZ).
Truncated-norzoanthamine (TZ) was synthesized as previously described [5]. 17 beta-estradiol (E2) was purchased from Wako Chemical Industries (Osaka, Japan). Pentobarbital sodium was purchased from Kyoritsu Seiyaku Corp. (Tokyo, Japan), and isoflurane was purchased from Intervet Inc. (Tokyo, Japan).
AnimalsThe female ddy mice used in this study were purchased from Japan SLC (Shizuoka, Japan). The mice were housed in a room maintained at 25 ± 1°C, with free access to food and water. All animal experiments performed were approved by the Animal Care and Use Committee of The University of Tokyo.
Osteoporosis mouse model and drug administrationTo induce osteoporosis, 4-week old female ddy mice were ovariectomized (OVX) or sham-operated (Sham) under pentobarbital and isoflurane anesthesia [7]. The mice were divided into 7 groups: Sham group, OVX control group, OVX 17 beta-estradiol (E2 0.08 mg/kg/day) group, and TZ (0.004, 0.02, 0.1, 0.5 mg/kg/day) groups. Sham and control groups were orally administered saline, TZ group was orally administered TZ (0.004, 0.02, 0.1, 0.5 mg/kg), and the E2 group was administrated E2 (0.08 mg/kg) intraperitoneally. Drug administration commenced from the day after ovariectomy, with drugs administered 5 days a week for 4 weeks. The mice were humanely killed by cervical dislocation under deep isoflurane anesthesia to collect uteri and femur bones for measurements. The dried left femur bones were weighed and morphologically observed. The right femur bones were measured using micro-computed tomography (CT).
Measurement and observation of organ weight and dry femur boneAfter the mice were sacrificed, the heart, kidneys, liver, and uterus were retrieved and weighed. Soft tissue attached to the left femur was trimmed off and the femurs were dried at 60°C for 24 hr. The dried femurs were measured for dry weight and polished using a fine grindstone. The ground femurs were then washed with water to eliminate bone marrow. The femurs were dried once again and observed using a microscope.
Quantitative micro-CTFollowing isolation from mice, the right femur bones were fixed with 4% paraformaldehyde. To assess the effect of TZ treatment on bone structure in the OVX mice, the fixed femur bones were analyzed by micro-CT (InspeXio; Shimazu Science East Corp., Tokyo, Japan). Micro-CT was used to scan the trabecular and cortical bone volume within the metaphysis of the femur. The metaphysis area of the femur was set as the area to be measured, 1 to 2.5 mm above the growth plate. A threshold of 315 mg/cm3 was used for all evaluations. Standard bone structure parameters, including trabecular bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and bone mineral density (BMD) were calculated using the TRI/3D Bon software (RATOC System Engineering Co., Ltd., Tokyo, Japan).
Clinical blood testAt the end of E2 or TZ administration, the mice were anesthetized and exsanguinated to obtain heparinized whole blood. After centrifugation of the whole blood, plasma was used to measure calcium, inorganic phosphorus, and alkaline phosphatase (ALP) in the blood of OVX mice treated with TZ or E2 (Fuji Dri-chem 7000v, Fujifilm, Tokyo, Japan).
Mechanical analysisFollowing the micro-CT examination, bone biomechanical parameters were measured using the Instron® mechanical test system (Instron-3365; Instron Corp., Norwood, MA, USA). The femurs were examined using the three-point bending until collapse. The femur was placed in the AP position on the lower supports of the bending apparatus, located at every 3.5 mm from the femur midpoint. The upper loading point was aligned to the midpoint of the femur. The actuator was displaced vertically downward at the rate of 50 mm/min until failure. The failure load and the yield energy were determined from the mechanical test recordings. The stiffness was calculated from the slope of the linear portion of the load-displacement curve.
Statistical analysisAll data were expressed as the mean ± S.E.M. of the indicated number of independent experiments. Statistical significance was determined by Dunnett’s test. A P-value <0.05 was considered statistically significant.
TZ or E2 administration did not alter the heart, liver, kidney, and body weights (Fig. 2A–D). Although E2 significantly suppressed the decrease in uterine weight induced following ovariectomy, TZ did not affect the uterine weight (Fig. 2E). This indicated that TZ did not have an estrogen-like effect on the reproductive organ. Additionally, TZ or E2 treatment demonstrated no difference in calcium, inorganic phosphorus, and ALP blood levels in OVX mice when compared with the vehicle-treated group (Table 1).
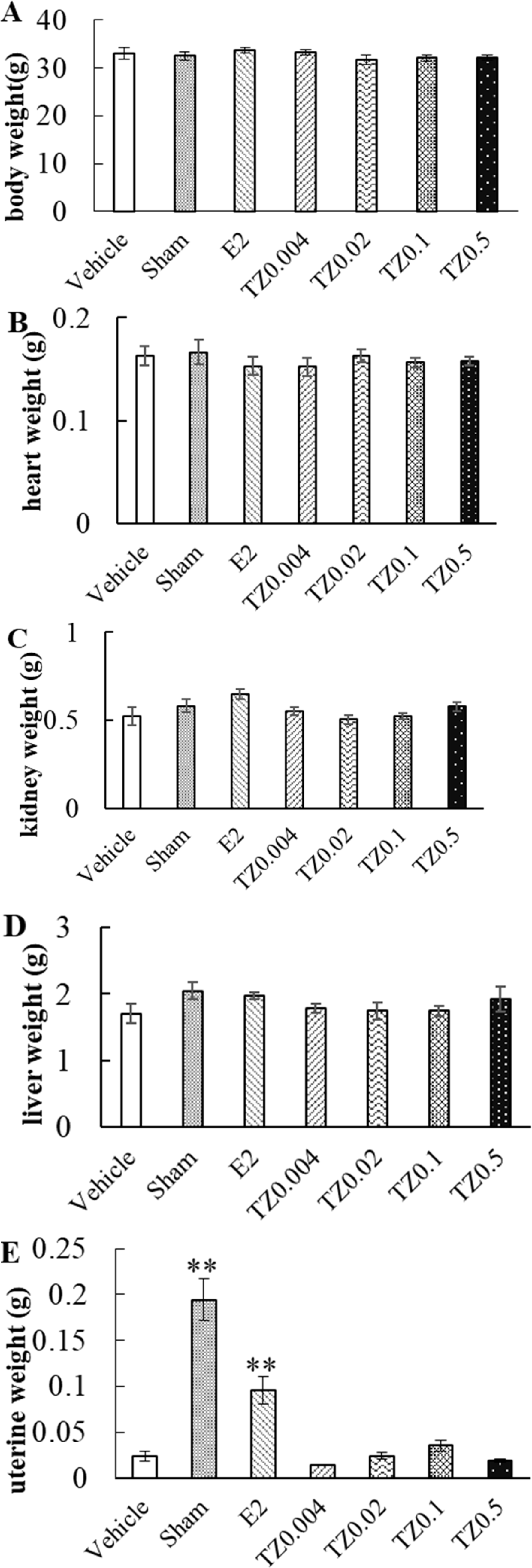
Effect of truncated-norzoanthamine (TZ) on body weight and organ weight in ovariectomized (OVX) mice. Each group of OVX mice were administered saline, E2 (0.08 mg/kg), or TZ (0.004, 0.02, 0.1, 0.5 mg/kg). A) Body weight, B) heart weight, C) kidney weight, D) liver weight, and E) uterine weight were measured 4 weeks after surgery. Vehicle (n=5): negative control group, Sham (n=5): sham-operated group, E2 (n=5): E2 treated group, TZ0.004 (n=8), TZ0.02 (n=8), TZ0.1 (n=8), TZ0.5 (n=4): TZ treated groups. Each group was expressed as mean ± S.E.M., **P<0.01.
| Vehicle | Sham | E2 | TZ0.02 | TZ0.004 | |
|---|---|---|---|---|---|
| IP (mg/dl) | 10.00 ± 0.47 | 9.75 ± 1.16 | 10.16 ± 0.55 | 12.06 ± 0.50 | 11.91 ± 0.59 |
| Ca (mg/dl) | 11.27 ± 0.44 | 11.50 ± 0.49 | 10.82 ± 0.38 | 11.18 ± 0.26 | 11.11 ± 0.37 |
| ALP (U/l) | 380.75 ± 34.03 | 438.00 ± 64.41 | 318.20 ± 35.34 | 413.80 ± 41.10 | 418.83 ± 24.00 |
Each group was expressed as mean ± S.E.M. n=5.
The femoral dry weight was not altered following TZ administration when compared with the vehicle and sham groups. Furthermore, E2 did not affect the femoral dry weight (Fig. 3A). The femoral trabecular bone decreased grossly following ovariectomy (Fig. 3B). In the femur, TZ suppressed the loss of trabecular bone. In particular, the bone amount of the secondary spongiosa did not decrease following TZ administration, as well as E2 treatment (Fig. 3B). The results demonstrated that TZ would provide a protective effect mainly on the trabecular bone.
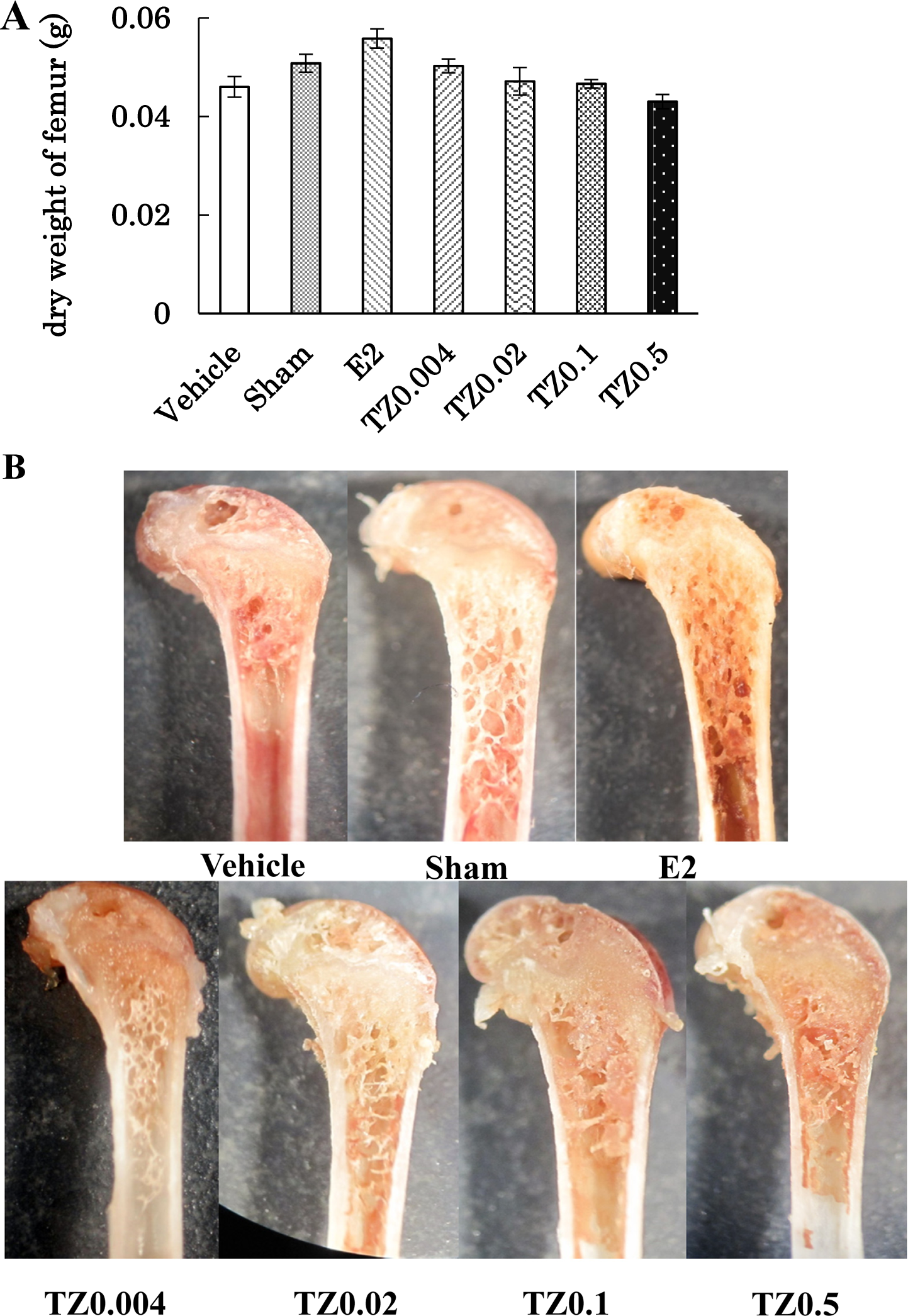
Effect of truncated-norzoanthamine (TZ) on the femur bone in ovariectomized (OVX) mice. Each group of OVX mice were administered saline, E2 (0.08 mg/kg), or TZ (0.004, 0.02, 0.1, 0.5 mg/kg). Vehicle (n=5): negative control group, Sham (n=5): sham-operated group, E2 (n=5): E2 treated group, TZ0.004 (n=8), TZ0.02 (n=8), TZ0.1 (n=8), TZ0.5 (n=4): TZ treated group. A) Dry weight of femur was measured 4 weeks after surgery. Each group was expressed as mean ± S.E.M. B) Morphological changes are observed in the dry femur of OVX mice.
To assess the effect of TZ treatment in OVX mice, we examined bone structures by micro-CT. In the OVX mice, low dose TZ (TZ 0.004 and 0.02 mg/kg/day p.o.) significantly increased BV/TV and Tb.N. when compared with the negative control group (vehicle) (Fig. 4A, 4C). Although Tb.Th. of E2 treated group significantly increased when compared with that of vehicle, TZ treatment failed to improve Tb.Th. in the OVX mice (Fig. 4B), suggesting that TZ treatment suppressed the loss of trabecular number induced by osteoporosis, preserving bone volume. In addition to these results, the BMD of trabecular bone were also improved in TZ groups (Fig. 4D).
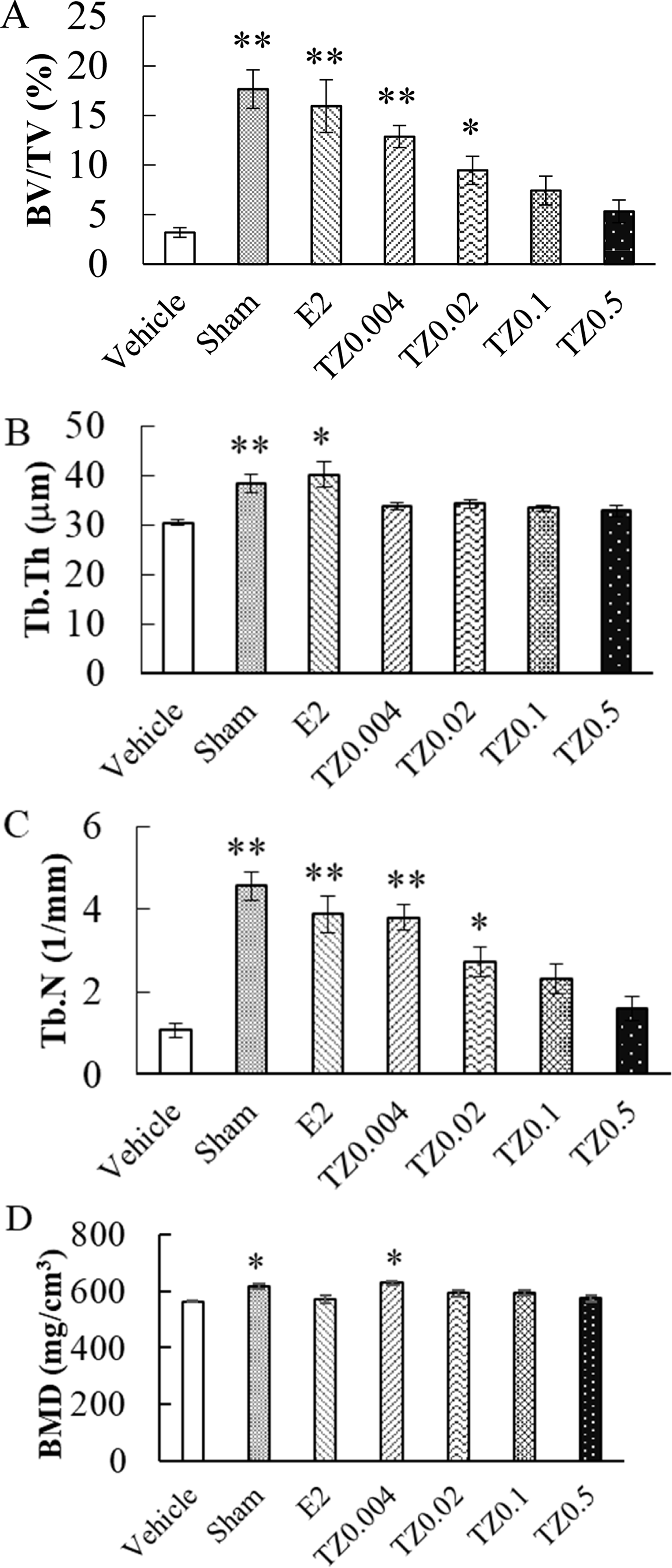
Effect of truncated-norzoanthamine (TZ) on BV/TV, Tb.Th, Tb.N, and bone mineral density (BMD) of femur bone. Each group of ovariectomized (OVX) mice were administered saline, E2 (0.08 mg/kg), or TZ (0.004, 0.02, 0.1, 0.5 mg/kg). The femur bones were collected from OVX mice after the sacrifice. Vehicle (n=5): negative control group, Sham (n=5): sham-operated group, E2 (n=5): E2 treated group, TZ0.004 (n=8), TZ0.02 (n=8), TZ0.1 (n=8), TZ0.5 (n=4): TZ treated groups. A) BV/TV, B) Tb.Th, C) Tb.N, and D) BMD were measured by micro-CT. Each point was expressed as mean ± S.E.M. **P<0.01. *P<0.05. BV/TV, trabecular bone volume; Tb.Th, trabecular thickness; Tb.N, trabecular number; BMD, bone mineral density.
To examine whether TZ treatment could improve bone strength, bone mechanical parameters (failure load, yield energy, and stiffness) were measured in the femur. Compared with the vehicle, TZ treatment increased the failure load induced by ovariectomy, although E2 treatment suppressed this parameter (Fig. 5A). However, regarding yield energy and stiffness, TZ treatment improved both indexes similar to E2 treatment (Fig. 5B, 5C).
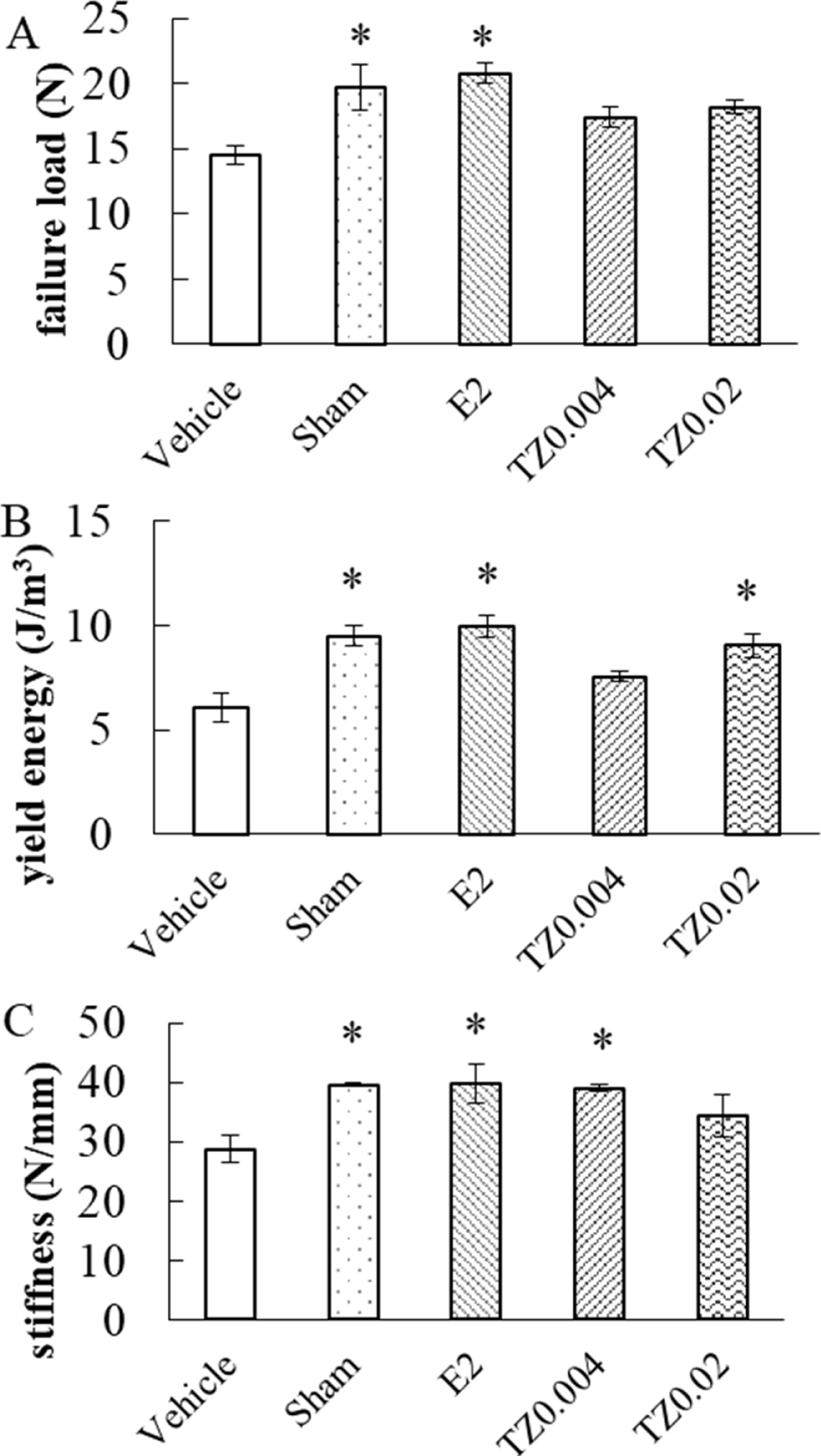
Effect of truncated-norzoanthamine (TZ) on bone mechanical parameters in ovariectomized (OVX) mice. Each group of OVX mice were administered saline, E2 (0.08 mg/kg), or TZ (0.004, 0.02 mg/kg). Vehicle (n=5): negative control group, Sham (n=5): sham-operated group, E2 (n=5): E2 treated group, TZ0.004 (n=5), and TZ0.02 (n=5): TZ treated group. A) Failure load, B) yield energy, and C) stiffness were measured. Each point was expressed as mean ± S.E.M. *P<0.05.
OVX mice have been used as an osteoporosis animal model due to the decreasing bone volume and bone mechanical parameters observed in this model. Reportedly, NZ administration suppresses bone loss and maintains bone mechanical parameters [3, 7]. Compared with the sham group, bone parameters (BV/TV, Tb.Th., Tb.N.) were significantly decreased in the control group (Figs. 3 and 4). TZ significantly suppressed the decrease of BV/TV and BMD by preserving Tb.Th. in the trabecular bone (Fig. 4). Furthermore, organ weight, body weight, and biochemical parameters were not affected following TZ administration (Fig. 2, Table 1), suggesting that TZ may have significant suppressive effects in osteoporosis without considerable side effects.
Currently, several drugs have already been used for the treatment of patients with osteoporosis; the mechanism of most drugs involves the suppression of osteoclast activity. E2 inhibits bone resorption and increases bone mass by regulating the life span of osteoclasts [8]. Osteoclasts suppression can maintain bone density at a high level and preserve the mature bone. In the case of bone morphology, TZ suppressed trabecular bone loss; E2 demonstrated similar effects (Fig. 3B). Regarding bone architecture, E2 significantly improved BV/TV, Tb.Th, and Tb.N. in the OVX model [9]. However, while E2 improved Tb.Th, TZ failed to demonstrate an improvement in this parameter (Fig. 4). The other anti-osteoporosis drug, the parathyroid hormone (PTH), reportedly improves Tb.N and Tb.Th in the OVX model [10]. PTH mainly acts by activating osteoblast. However, in osteoporosis, the effects of PTH are substantially weaker when compared with E2 [11]. In osteoporosis treatment, osteoclast suppression is generally considered more effective than osteoblast activation. Based on this perspective, the results of this study evoke the possibility that TZ could play a novel role in contrast to osteoclast suppression.
Among the bone mechanical parameters, failure load directly indicates the cortical bone strength. In this study, TZ failed to suppress the decrease in ovariectomy-induced failure load, suppressed following treatment with E2. TZ improved the yield energy and stiffness (Fig. 5), indicating that TZ may act on the cortical bone rather than the trabecular bone. Bone mechanical strength is one of the most important parameters used for assessing drug efficacy. In osteoporosis, this parameter predicts the risk of bone fracture. In the present study, the results demonstrated that TZ may clinically improve osteoporosis and prevent pathological fractures. Furthermore, drugs used in the treatment of osteoporosis are preferably oral drugs owing to chronic usage. Hence, TZ could meet this requirement.
In the micro-CT and bone mechanical test results, the optimum concentration of TZ was not shown. Notably, the lowest TZ dose (0.004 mg/kg) demonstrated marked bioactivity when compared to the highest dose. A larger TZ dose could induce the downregulation of osteogenic factors. In comparison with a previous report on NZ (0.02 mg/kg), this TZ dose was more potent and effective [3]. Furthermore, it was less likely that the analogous compounds reported equivalent efficacy as natural compounds. Therefore, we believe that TZ could be developed as a novel anti-osteoporosis drug.
In this study, TZ suppressed bone loss in the osteoporosis model, but the mechanism of TZ remains unclear. Reportedly, NZ, possessing a similar structure to TZ, suppresses in vitro interleukin-6 production in MC3T3-E1 cells [12]. Interleukin-6 is related to osteoclast formation. However, the suppression of interleukin-6 following the NZ administration has not been confirmed in vivo [3]. In another report, NZ increased the total collagen in MC3T3-E1 cells and inhibited collagenase activity [4]. Additionally, it was confirmed that TZ could also inhibit collagenase activity [6]. Therefore, TZ could increase total collagen similar to NZ . Reportedly, collagen cross-linking plays an important role in determining bone quality [13]. It is postulated that lysyl oxidase increases mature cross-linking in physiological collagen cross-linking [14]. TZ may also be involved in collagen cross-linking. We will examine the effects of TZ on the total collagen and collagen cross-linking in osteoblastic cells and investigate the target molecule of TZ.
We present the possibility that TZ is a new drug for the treatment of osteoporosis. Furthermore, elucidating the mechanism of TZ could provide innovative ideas regarding possible anti-osteoporosis strategies.