2021 Volume 3 Issue 1 Pages 1-8
2021 Volume 3 Issue 1 Pages 1-8
Human epidermal growth factor receptor 2 (HER2) is a cell surface receptor tyrosine kinase that belongs to the HER family. HER2 overexpression in human prostate cancer has been frequently observed; however, there are no reports on HER2 expression in canine prostate carcinoma (PC). The purpose of this study was to investigate HER2 expression and the underlying molecular mechanisms in canine PC. Twenty-one formalin-fixed paraffin-embedded canine prostate gland tissues (13 carcinomas and 8 normal controls) were analyzed for HER2 expression using immunohistochemistry. Using a digital polymerase chain reaction assay, the HER2 copy number in 8 PC and 6 normal prostate gland tissues was determined. HER2 overexpression occurred in 8/13 (61.5%) PC cases, but not in the normal controls. There were no significant associations between HER2 overexpression and clinical characteristics of the PC cases, such as age, neuter status, lymph node involvement, distant metastasis, and overall survival. HER2 copy number gain (CNG) was detected in 3/8 (37.5%) PC cases, but not in the normal controls. All PC cases with HER2 CNG exhibited HER2 overexpression, suggesting that HER2 CNG may lead to HER2 overexpression. These findings provide new insights into the molecular pathogenesis of canine PC; these may be therapeutically relevant. Dogs with PC would be useful as a preclinical large animal model for the development of new therapy for HER2-overexpressing prostate cancer in humans.
● HER2 overexpression was observed in 61.5% of canine PC cases, suggesting that HER2 can be a therapeutic target in canine PC.
● Since all canine PC cases with HER2 CNG exhibited HER2 overexpression, HER2 CNG may be one of the mechanisms underlying HER2 overexpression.
● Dogs with HER2-overexpressing PC may be a novel research model for HER2-overexpressing prostate cancer in humans.
Prostate carcinoma (PC) is a malignant tumor originating in the prostate glands. Although this is one of the most common cancers in humans, it is relatively rare in dogs. However, the biological behavior of canine PC is extremely aggressive. Generally, this condition affects older dogs, with an average age of occurrence of approximately 10 years [1[[. Castrated males have a higher risk of developing PC than intact males [3]. The breed disposition is not clear; however, a study has shown that Bouvier des Flandres has an approximately 8.5 times higher risk of developing PC than other breeds [3]. Hematuria, stranguria, and tenesmus are common clinical symptoms of dogs with PC. These symptoms are also observed in dogs with non-PC diseases such as cystitis and prostatitis, often delaying the diagnosis of PC. Hence, most dogs with PC present with advanced stages of the disease at the time of diagnosis [1, 4]. Surgery, radiation therapy, and chemotherapy are routinely used to treat dogs with PC [5]. Since the success of surgery and radiation therapy is limited due to the high rate of local invasion and metastasis, chemotherapy is considered the mainstay for the treatment of canine PC. However, there is no universally accepted protocol, and unfortunately, most dogs with PC are euthanized at the time of diagnosis. In one study, the median survival for 76 dogs with PC was 0 days [1]. Therefore, the development of effective medical therapy for dogs with PC is urgently needed.
Human epidermal growth factor receptor 2 (HER2), also known as avian erythroblastosis oncogene B 2 (ErbB2), is a cell surface receptor tyrosine kinase that belongs to the HER family (HER1–4) and is involved in cell proliferation, survival, angiogenesis, and migration [6]. There are many reports on molecular abnormalities in a variety of human cancers such as prostate, breast, bladder, and gastric cancers [7,8,9,10,11,12,13,14,15,16]. Under normal circumstances, a cell has two copies of HER2; however, in human cancers, this gene is amplified to more than two copies, a phenomenon known as HER2 copy number gain (CNG) [9, 10, 12,13,14]. HER2 CNG is caused by gene amplification and/or polysomy, and leads to HER2 protein overexpression [9, 12,13,14]. Human patients with HER2-overexpressing cancers show poor prognosis [7, 8, 11, 15, 16]. In addition to being a prognostic factor, HER2 is an important therapeutic target. HER2-targeted therapy, including drugs such as trastuzumab and lapatinib, has improved the survival times of human patients with HER2-overexpressing breast and gastric cancers [17,18,19,20,21]. Given its prognostic and therapeutic implications, the assessment of HER2 levels in human patients with these cancers is crucial.
As in humans, HER2 overexpression has been observed in canine malignant tumors such as transitional cell carcinoma, mammary carcinoma, gastric carcinoma, anal sac carcinoma, thyroid carcinoma, and osteosarcoma [22,23,24,25,26,27]. Moreover, our previous study, using canine transitional cell carcinoma cell lines and cell line–engrafted mice, indicated that HER2-targeted therapy has therapeutic potential in dogs with transitional cell carcinoma [28]. Mason et al. (2016) have shown an anti-tumor effect of the recombinant Listeria-HER2 vaccine in dogs with osteosarcoma [22]. These findings suggest that HER2 is an important therapeutic target for malignant tumors, not only in humans but also in dogs. However, there are no reports on HER2 expression in canine PC.
The purpose of this study was to examine HER2 expression in canine PC and investigate the relationship between HER2 overexpression and HER2 CNG.
From an archive of formalin-fixed paraffin-embedded (FFPE) canine prostate gland tissues, 13 tumor tissue samples from dogs with PC were used for study (Supplementary Table 1). These dogs underwent total cystoprostatectomy (n=7), total prostatectomy (n=4), or biopsy (n=2) at the Veterinary Medical Center of The University of Tokyo from November 2009 to August 2017. All the dogs were males (11 castrated and 2 intact), with a median age of 139 months (range: 108–151 months). The cases represented a mix of different breeds. Lymph node involvement was observed in 3 cases, and distant metastasis was observed in another three. Neither lymph node involvement nor distant metastasis was observed in 7 cases. Eight tissue samples from normal prostate glands were used as controls (Supplementary Tabl[. All the dogs were intact males, with a median age of 132 months (range: 72–132 months). The breeds included Beagle (n=7) and Miniature Schnauzer (n=1).
Urine samples were collected using urethral catheters from 31 dogs with PC enrolled at the Veterinary Medical Center of The University of Tokyo from July 2015 to May 2019 (Supplementary Table 2). All the dogs were males (28 castrated and 3 intact), with a median age of 144 months (range: 85–188 months). The cases represented a mix of different breeds. Out of the 31 dogs, seven were diagnosed by histopathological evaluation of excised mass lesions, whereas 24 dogs were diagnosed based on clinical signs, presence of prostate swelling in ultrasound, and cytological evidence of abnormal epithelial cells in urinary sediments. Urine samples from 10 clinically healthy dogs were used as normal controls (Supplementary Table 2). The normal controls were the same samples used in our previous study [29]. Informed consent for the use of the clinical data and samples was obtained from the dog owners. This study did not reach the threshold for submission to a local ethical and welfare committee, since the collection and use of clinical data and samples were daily activities.
ImmunohistochemistryHER2 expression was examined using immunohistochemistry according to a protocol described in our previous report [28]. Briefly, 4 µm sections were cut from FFPE canine prostate gland tissues. These sections were de-paraffinized in xylene and hydrated in graded ethanol. Antigen retrieval was performed at 121°C for 10 min using 10 mM citrate buffer (pH 6.0). After cooling with running water for 30 min, the slides were treated with a peroxidase-blocking solution (Dako, Glostrup, Denmark) at room temperature (26°C) for 10 min, followed by three washes in Tris-buffered saline (TBS) with 0.1% Tween 20 (TBS-T). After blocking with TBS-T containing 5% skim milk (Wako, Osaka, Japan) for 1 hr, the sections were incubated with rabbit polyclonal anti-human c-erbB-2 oncoprotein (HER2/neu) antibodies (diluted 1:100 in TBS, Dako) at 37°C for 40 min. The sections were subsequently incubated with Envision horseradish peroxidase-labeled anti-rabbit IgG polymer (Dako) at 37°C for 40 min. The peroxidase reaction was conducted using 3,3ʹ-diaminobenzidine tetrahydrochloride (Dako) for 10 min. The reaction was stopped with two washes in distilled water and the sections were counterstained with Mayer’s hematoxylin. The positive control was a canine transitional cell carcinoma tissue, which is known to overexpress HER2 [25]. Sections incubated with antibody diluents alone served as the negative controls.
HER2 immunoreactivity was quantified based on our previous study [25]. A score of 0 indicated no reactivity, a score of 1+ represented incomplete and weak immunoreactivity in <10% of the tumor cells, a score of 2+ represented incomplete but intense immunoreactivity in ≥10% of the tumor cells, and a score of 3+ represented intense and complete immunoreactivity in ≥10% of the tumor cells. Samples with scores of 0 and 1+ were classified as HER2 overexpression-negative, and those with scores of 2+ and 3+ were classified as HER2 overexpression-positive. Although cytoplasmic immunoreactivity was detected in some tumor cells and normal prostate epithelial cells, these were not included in the analyses.
DNA isolation and digital polymerase chain reaction (PCR)Tissue DNA from 8/13 PC samples and 6/8 normal controls was isolated using the QIAmp DNA FFPE Tissue Kit (QIAGEN, Hilden, Germany). Urinary DNA was isolated from all dogs, using the DNeasy Blood and Tissue Kit (QIAGEN). A digital PCR assay was performed to assess the HER2 copy number. This assay was established in our previous study [29]. Briefly, primers and TaqMan minor groove binder probes for HER2, and a reference gene were designed with the Primer Express Software (Thermo Fisher Scientific, Waltham, MA, USA). Since canine HER2 is located on chromosome 9, the reference gene was selected from chromosome 8 (CFA8). The primer and probe sequences were as follows: HER2 forward, 5ʹ-GTGGTGAGGCTGGTTTTCAGA-3ʹ; HER2 reverse, 5ʹ-CCTGTCCTCCCACCTCTTCAT-3ʹ; HER2 probe, 5ʹ -TAACCGCTAAGCAGTATGT-3ʹ; CFA8 forward, 5ʹ-TGCAGAGTTTGATTTGTTGTTTGAA-3ʹ; CFA8 reverse, 5ʹ -TGGAAGAAGGTGCATTTTTCTGA-3ʹ; CFA8 probe, 5ʹ -AATGCCTTTGACCAGTGGGTAGCC-3ʹ. The HER2 and CFA8 probes were labeled with 6-carboxyfluorescein and 4,7,2ʹ-trichloro-7ʹ-phenyl-6-carboxyfluorescein, respectively. All primers and probes were custom-made by Thermo Fisher Scientific. PCR was performed using a QuantStudio 3D Digital PCR system (Thermo Fisher Scientific). Briefly, each 15-µl reaction mixture contained 2× QuantStudio 3D Digital PCR Master Mix v2 (Thermo Fisher Scientific), 900 nM of each primer, 200 nM of each probe, and 20 ng of genomic DNA from FFPE canine prostate gland tissues or 20–30 ng of genomic DNA from urinary sediments. The reaction mixtures were then loaded onto a QuantStudio 3D Digital PCR 20K Chip v2 (Thermo Fisher Scientific) using a QuantStudio 3D Digital PCR Chip Loader (Thermo Fisher Scientific). The 20K chip contained 20,000 individual wells, within which DNA was randomly and uniformly distributed. The chip containing the reaction mixture was subjected to thermocycling in a ProFlex 2× Flat PCR System (Thermo Fisher Scientific) under the following conditions: denaturation at 96°C for 10 min; and 39 cycles at 60°C for 2 min, 98°C for 30 sec, and 60°C for 2 min, after which the temperature was maintained at 10°C. After the digital PCR assay, the PCR chip was loaded onto a QuantStudio 3D Digital PCR Instrument (Thermo Fisher Scientific). The positive and negative plots were counted, and HER2:CFA8 ratios were calculated using Poisson’s distribution with QuantStudio 3D AnalysisSuite version 3.1.2 (Thermo Fisher Scientific).
Statistical analysesFor survival analysis, information regarding the current status (alive, dead, or lost) at the end of the study (May 22, 2019) was obtained for each PC case based on the medical records or a fax interview with referring veterinarians. Overall survival was defined as the interval between surgery and the established cause of death at the end of this study. Survival curves of the PC cases with and without HER2 overexpression were generated using the Kaplan–Meier method, and the survival rates were compared using the log-rank test.
A Fisher’s exact test was used to determine the association of HER2 expression with the clinical characteristics of PC cases. A Mann–Whitney U test was performed to determine differences in tissue and urinary HER2:CFA8 ratios between the normal controls and PC cases. Statistical analyses were performed using Prism software version 8.2.0 (Graph Pad Software, San Diego, CA, USA). Statistical significance was defined as P<0.05.
The immunohistochemical staining scores for HER2 expression in each sample are listed in Supplementary Table 1. Among normal prostate glands, 7/8 (87.5%) samples scored 0, and 1/8 (12.5%) scored 1+. No HER2 overexpression (score 2+ or 3+) was observed (Fig. 1A). In PC, HER2 immunoreactivity was heterogeneous within individual tumors. Five out of the 13 (38.5%) samples scored 0, 6/13 (46.2%) scored 2+, and 2/13 (15.4%) scored 3+. Eight out of the 13 (61.5%) samples were HER2 overexpression-positive (Fig. 1B), whereas 5/13 (38.5%) were HER2 overexpression-negative (Fig. 1C). HER2 expression in the metastatic skin lesion and primary tumor was examined for a PC case with metastasis. Both samples scored 2+ in HER2 immunoreactivity (Fig. 1D). For other cases with metastasis, tissue samples could not be obtained from the metastatic sites, as these were not excised during surgery.

Representative results of immunohistochemistry for human epidermal growth factor receptor 2 in canine prostate gland tissues. (A) Normal prostate glands showing no immunoreactivity (score 0). (B) Neoplastic cells of prostate carcinoma showing strong membranous immunoreactivity (score 3). (C) Neoplastic cells of prostate carcinoma showing no immunoreactivity (score 0). (D) Neoplastic cells of metastatic skin lesion as well as primary site in a case with prostate carcinoma showing strong membranous immunoreactivity (score 2). Samples were counterstained with Mayer’s hematoxylin. Scale bars=50 µm.
There was no significant association between HER2 expression and clinical characteristics of the PC cases, such as age, neuter status, lymph node involvement, distant metastasis, and overall survival (Table 1).
| HER2 overexpression | P value | ||
|---|---|---|---|
| Positive (n=8) | Negative (n=5) | ||
| Age (months)a | |||
| <Median | 5 | 2 | 0.5921 |
| ≥Median | 3 | 3 | |
| Neuter statusa | |||
| Yes | 7 | 4 | 1.0000 |
| No | 1 | 1 | |
| Lymph node involvementa | |||
| Yes | 2 | 1 | 1.0000 |
| No | 6 | 4 | |
| Distant metastasisa | |||
| Yes | 1 | 2 | 0.5105 |
| No | 7 | 3 | |
| Overall survivalb | |||
| 284 | 285 | 0.7455 | |
aData indicate the number of cases. bData indicate the median overall survival (days).
To elucidate the mechanism of HER2 overexpression, we performed digital PCR to detect HER2 CNG (Fig. 2A), as previously described [29]. The median HER2:CFA8 ratios in the normal controls and PC tissues were 0.91 (range, 0.74−0.98) and 1.05 (range, 0.74−1.91), respectively (Fig. 2B). The tissue HER2:CFA8 ratios in PC were significantly higher than those in the normal controls (P=0.0426). To determine a universal threshold of HER2 CNG in canine prostate gland tissues, the mean + 3 standard deviation of the tissue HER2:CFA8 ratios in the normal controls was calculated (1.18). At this threshold, HER2 CNG was detected in 3/8 (37.5%) PC cases, but not in the normal controls (Fig. 2B). For 8 PC cases, both copy number and protein expression of HER2 were evaluated. As shown in Table 2, HER2 CNG was detected in 3/7 (42.9%) PC cases with HER2 overexpression. HER2 overexpression was observed in all PC cases with HER2 CNG. For a PC case with metastasis, DNA samples were obtained from the primary tumor and metastatic skin lesion, and the HER2:CFA8 ratios were 1.31 and 1.21, respectively. HER2 CNG was detected in both the samples.
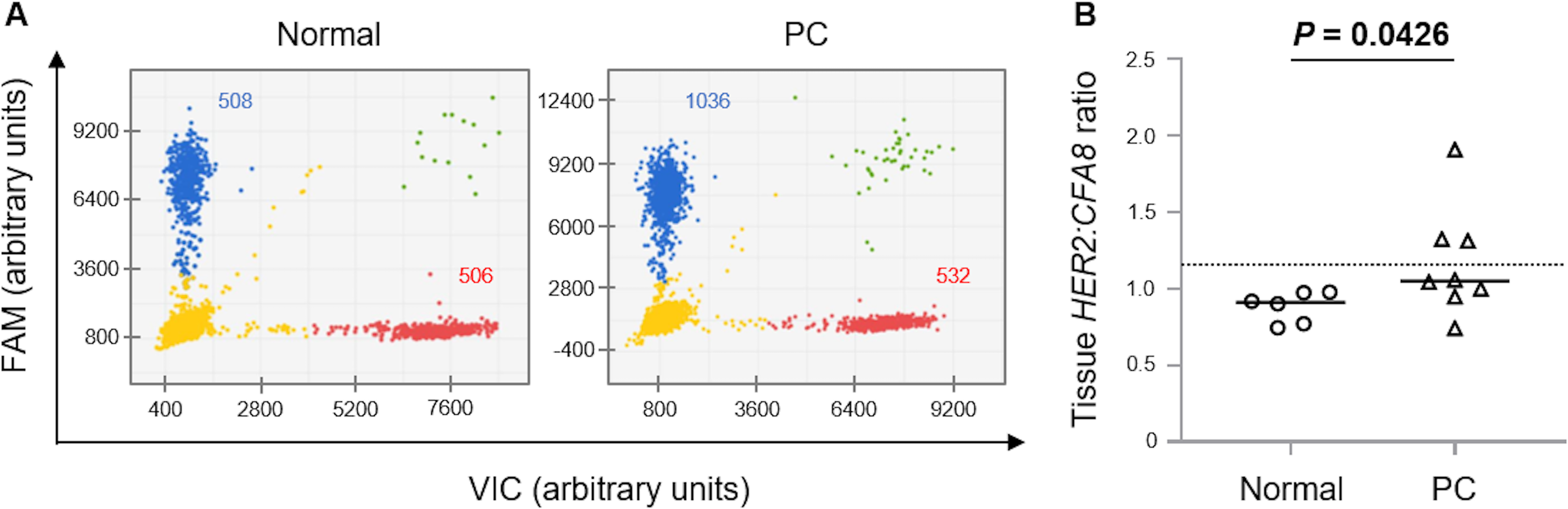
Digital polymerase chain reaction assay for detecting human epidermal growth factor receptor 2 (HER2) copy number in DNA samples obtained from canine prostate gland tissues. (A) Two-dimensional scatter plots of a normal control and a canine prostate carcinoma (PC) case. HER2 was labeled with 6-carboxyfluorescein (FAM), whereas the reference gene in chromosome 8 (CFA8) was labeled with 4,7,2ʹ-trichloro-7ʹ-phenyl-6-carboxyfluorescein (VIC). Four clusters were identified to be single-positive for FAM (blue) and VIC (red), double-positive (green), and double-negative (yellow). The ratio of blue plot points to red plot points in the PC case (1,036:532) was much higher than that in the normal control (508:506). (B) Tissue HER2:CFA8 ratios in canine normal prostate glands (n=6) and PC (n=8). The dotted line indicates a universal threshold (>1.18) for detecting HER2 copy number gain. The horizontal lines indicate the median.
| HER2 overexpression | ||
|---|---|---|
| Positive (n=7) | Negative (n=1) | |
| HER2 copy number gain | ||
| Positive (n=3) | 3 | 0 |
| Negative (n=5) | 4 | 1 |
Data indicate the number of cases.
To determine the HER2 copy number in urinary tumor cells, we performed digital PCR using urinary DNA (Fig. 3A). The median urinary HER2:CFA8 ratios in the normal controls and PC cases were 0.94 (0.79−0.99) and 0.99 (0.74−3.59), respectively (Fig. 3B). The urinary HER2:CFA8 ratios in the PC cases were significantly higher than those in the normal controls (P=0.0251). To determine a universal threshold of HER2 CNG in urinary sediments, the mean + 3 standard deviation of the HER2:CFA8 ratios in the normal controls was calculated (1.13). At this threshold, HER2 CNG was detected in 4/31 (12.9%) PC cases, but not in the normal controls (Fig. 3B).
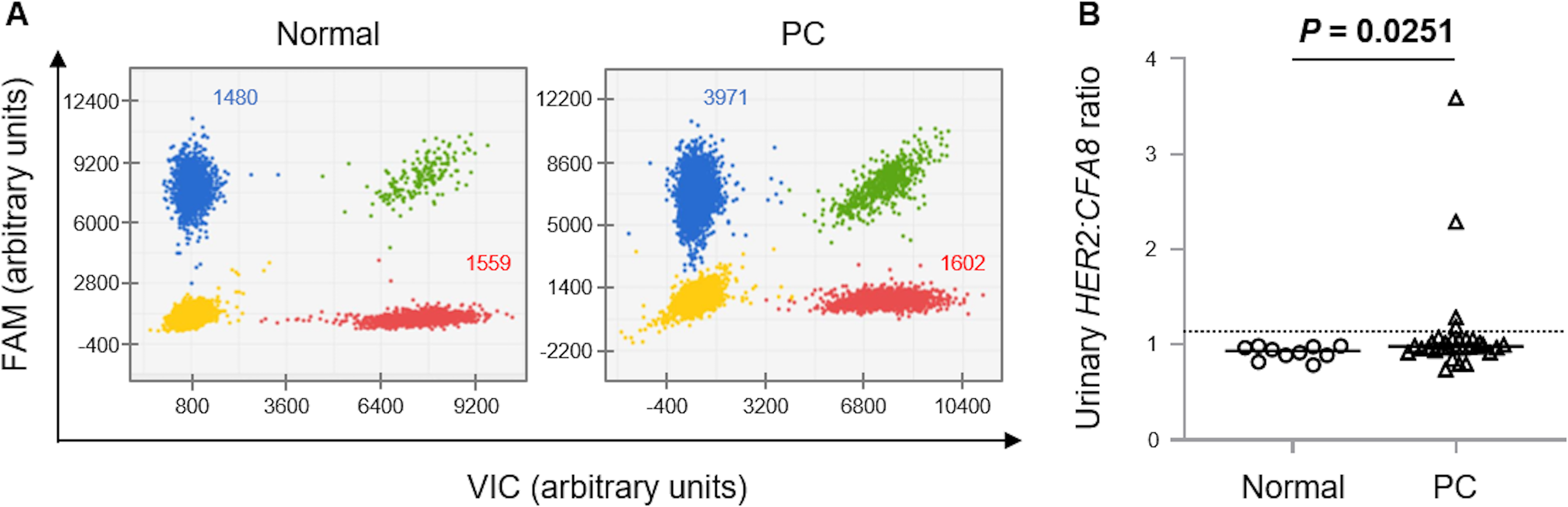
Digital polymerase chain reaction assay for detecting human epidermal growth factor receptor 2 (HER2) copy number in DNA samples obtained from canine urine. (A) Two-dimensional scatter plots for a normal control dog and a dog with prostate carcinoma (PC). HER2 was labeled with 6-carboxyfluorescein (FAM), whereas the reference gene in chromosome 8 (CFA8) was labeled with 4,7,2ʹ-trichloro-7ʹ-phenyl-6-carboxyfluorescein (VIC). Four clusters were identified to be single-positive for FAM (blue) and VIC (red), double-positive (green), and double-negative (yellow). The ratio of blue plot points to red plot points in the PC case (3,971:1,602) was much higher than that in the normal control (1,480:1,559). (B) Urinary HER2:CFA8 ratios in clinically healthy dogs (n=10) and dogs with PC (n=31). The dotted line indicates a universal threshold (>1.13) for detecting HER2 copy number gain. The horizontal lines indicate the median.
In this study, HER2 overexpression was observed in 61.5% of canine PC cases. Moreover, HER2 CNG was detected in 37.5% of PC cases. HER2 overexpression occurred in all PC cases with HER2 CNG, suggesting that HER2 CNG may lead to HER2 overexpression. In some PC cases, HER2 overexpression without HER2 CNG was observed. This finding indicates other mechanisms underlying HER2 overexpression in canine PC. In fact, it is known that HER2 overexpression in human breast cancer is caused by an increase in transcription through high levels of transcriptional activators, such as action protein 2 and Yin Yang 1 [30]. Further studies are needed to investigate in detail the mechanisms of HER2 overexpression in canine PC.
Human patients with HER2-overexpressing prostate cancer show poor clinical prognosis [7, 8]. In this study, however, there was no significant association between HER2 expression and overall survival in canine PC cases. There are some potential explanations for this discrepancy. Firstly, our sample size was small. Secondly, different clinical stages of canine PC cases were analyzed. Thirdly, the operative procedures and postoperative therapy in the canine PC cases were not unified. These differences may have influenced the overall survival in each case. Further studies using a larger cohort with a standardized therapy are needed to investigate the association of HER2 expression with prognosis of dogs with PC.
In addition to being a prognostic factor, HER2 is an important therapeutic target in human patients with breast and gastric cancers [17,18,19,20,21]. As in humans, several studies have shown that HER2-targeted therapy has therapeutic potential in dogs with malignant tumors such as transitional cell carcinoma and osteosarcoma [22, 28]. In this study, we found that HER2 overexpression occurred in canine PC. This molecular abnormality was present in both the primary and metastatic lesions of a dog with PC. Therefore, HER2 may be a useful therapeutic target for dogs with PC. In addition, HER2 CNG, which may lead to HER2 overexpression, was detected in the urinary sediment of dogs with PC. The digital PCR assay for detecting HER2 CNG in urinary sediments may be a useful method for predicting the efficacy of HER2-targeted therapy.
It has been proposed that canine PC is an important animal model for translational research to study human prostate cancer [4, 31, 32]. There are many similarities and a few differences between human prostate cancer and canine PC. Firstly, human and canine prostate glands share common embryonic development characteristics and exhibit homologous gross and microscopic anatomy [4]. Canine PC shows alterations in a set of genes similar to those in human prostate cancer [33]. In addition, dogs with PC often develop bone metastases of the pelvis and/or lumbar spine, similar to humans [4]. In contrast, the incidence of these tumors differs between the two species. Although PC is relatively rare in dogs, prostate cancer is the second most frequently diagnosed cancer in men worldwide, with 1.1 million new cases estimated to have occurred in 2012 [34]. Another difference between the two species is the degree of androgen-responsiveness. Most early-stage human prostate cancers are highly dependent on androgen as a growth factor [4]. Therefore, androgen deprivation is a foundational therapy for human prostate cancer as it provides marked clinical improvement. However, most canine PCs do not express androgen receptors. These findings suggest that PC in dogs represents a good model for the study of advanced, androgen-independent prostate cancer in humans. In this study, HER2 overexpression was detected in canine PC as well as human prostate cancer. Therefore, we suggest that dogs with HER2-overexpressing PC may be useful as a preclinical model for the development of a new therapy for HER2-overexpressing prostate cancer in humans.
There were some limitations to this study. Firstly, the sample size was small. Secondly, fluorescence in situ hybridization analysis to detect HER2 CNG was not performed on canine PC tissue samples. However, previous studies have shown that digital PCR assay data have a high concordance with the results of fluorescence in situ hybridization analysis of human breast cancer tissue samples [35, 36].
Our study demonstrated HER2 overexpression in canine PC. HER2 CNG may be one of the mechanisms underlying HER2 overexpression. Our findings provide new insights into the molecular pathogenesis of canine PC and therapy for dogs with this condition.
The authors declare no conflict of interest.
Canine prostate gland tissue samples were provided by Dr. Kazuyuki Uchida and Dr. James K. Chambers. Canine urine samples were kindly gifted by Dr. Ryohei Nishimura, Dr. Takayuki Nakagawa, and Dr. Kohei Saeki. This study was supported by the Grants-in-Aid for Scientific Research (KAKENHI Grant Numbers 16H06208 and 19H00968), fellows of the Japan Society for the Promotion of Science (JSPS), and the Anicom Capital Research Grant (EVOLVE).