2022 Volume 4 Issue 1 Pages 1-4
2022 Volume 4 Issue 1 Pages 1-4
Neutralizing antibodies against the receptor-binding domain of the spike protein play an important role in protecting SARS-CoV-2 infection. However, infectivity-enhancing antibodies recognizing the N-terminal domain of the spike are also produced following infection with SARS-CoV-2.
Antibodies against the receptor-binding domain (RBD) of the spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) play an important role in neutralizing antibodies that block the binding of the human receptor, angiotensin-converting enzyme 2 (ACE2), thereby suppressing SARS-CoV-2 infection [1,2,3]. In fact, various recent SARS-CoV-2 variants have acquired mutations in the epitopes of neutralizing antibodies, probably because the antibodies play an essential role in eliminating the viruses [4, 5]. On the other hand, in addition to the neutralizing antibodies, many antibodies against spike proteins are produced in patients with coronavirus disease (COVID-19), but the detailed functions of these antibodies have not yet been clarified [1,2,3].
As described above, antibodies play an important role in protecting against viral infections; however, some antibodies are known to exacerbate the infection, which is a phenomenon known as antibody-dependent enhancement (ADE). ADE has been observed in viruses such as the dengue virus and feline infectious peritonitis virus. Once infected by a serotype of dengue virus, infection with another serotype of that virus can cause severe disease due to the antibodies produced by the initial infection [6]. In addition, antibodies to the virus have been reported to be an exacerbating factor in feline infectious peritonitis virus, a family of coronavirus [7, 8]. The enhancement of infection by these antibodies is thought to involve Fc receptors expressed by certain types of immune cells. Thus, binding of virus-antibody immune complexes to cellular Fc receptors triggers virus entry into the cells. However, these Fc receptor-mediated infections are not significantly associated with the infection of many cells in the body, as the Fcreceptor expression is restricted to specific immune cells such as B cells and macrophages. To elucidate the function of antibodies produced in COVID-19 patients, we analyzed 76 clones of patient-derived recombinant monoclonal antibodies against the spike protein to assess their function in detail. We found a novel mechanism of ADE that is quite distinct from the previously known Fc receptor-mediated mechanism. Several monoclonal antibodies enhance the infectivity of SARS-CoV-2 in an Fc receptor-independent manner by simply binding to virus particles [9].
In SARS-CoV-2, the spike protein comprises N-terminal domain (NTD), RBD, and S2. RBD binds to the host cell receptor, ACE2, which triggers membrane fusion; subsequently, the virus infects the host cell [10, 11]. Once infected with SARS-CoV-2, antibodies against various sites of the spike protein are produced by the host’s immune response. The 76 antibodies to the spike proteins identified from immune cells of COVID-19 patients were analyzed. The analysis revealed that most of the antibodies to the RBD inhibited the binding of the spike proteins to ACE2. In contrast, antibodies against S2 did not affect the binding of ACE2 to the RBD. Surprisingly, it was proved that among the antibodies against the NTD, six increased the binding of ACE2 to the RBD [9]. Hence, these antibodies possibly enhance the infectivity of SARS-CoV-2, and they are referred to as “infectivity-enhancing antibodies”.
Neutralizing antibodies against the RBD inhibit the binding of ACE2 to spike proteins, whereas infectivity-enhancing antibodies attenuate this inhibition ability of the neutralizing antibodies. In other words, the production of infectivity-enhancing antibodies may result in the poor efficacy of neutralizing antibodies. Infection experiments have shown that infectivity-enhancing antibodies significantly enhance the infectivity of SARS-CoV-2 in human cells. The enhanced infectivity of these antibodies is a direct effect of the antibodies on the spike protein, and the Fc receptor is not involved. Therefore, it was found that there are antibodies that enhance infectivity by a new mechanism that is completely different from the previously known ADE.
Next, to clarify the recognition site of infectivity-enhancing antibodies, competitive inhibition experiments were conducted using the six types of infectivity-enhancing antibodies. The results showed that infectivity-enhancing antibodies recognize the adjacent sites of the NTD, because these antibodies competitively inhibit each other. The epitopes of infectivity-enhancing antibodies were then analyzed by replacing the amino acids of NTD with alanine. The results revealed that all infectivity-enhancing antibodies recognized specific sites in the NTD (Fig. 1, left image). Furthermore, cryo-electron microscopy was used to analyze the binding mode of the antibody-spike protein complexes, and the analysis revealed that the infectivity-enhancing antibody binds to the lower part of the NTD (Fig. 1, right image).
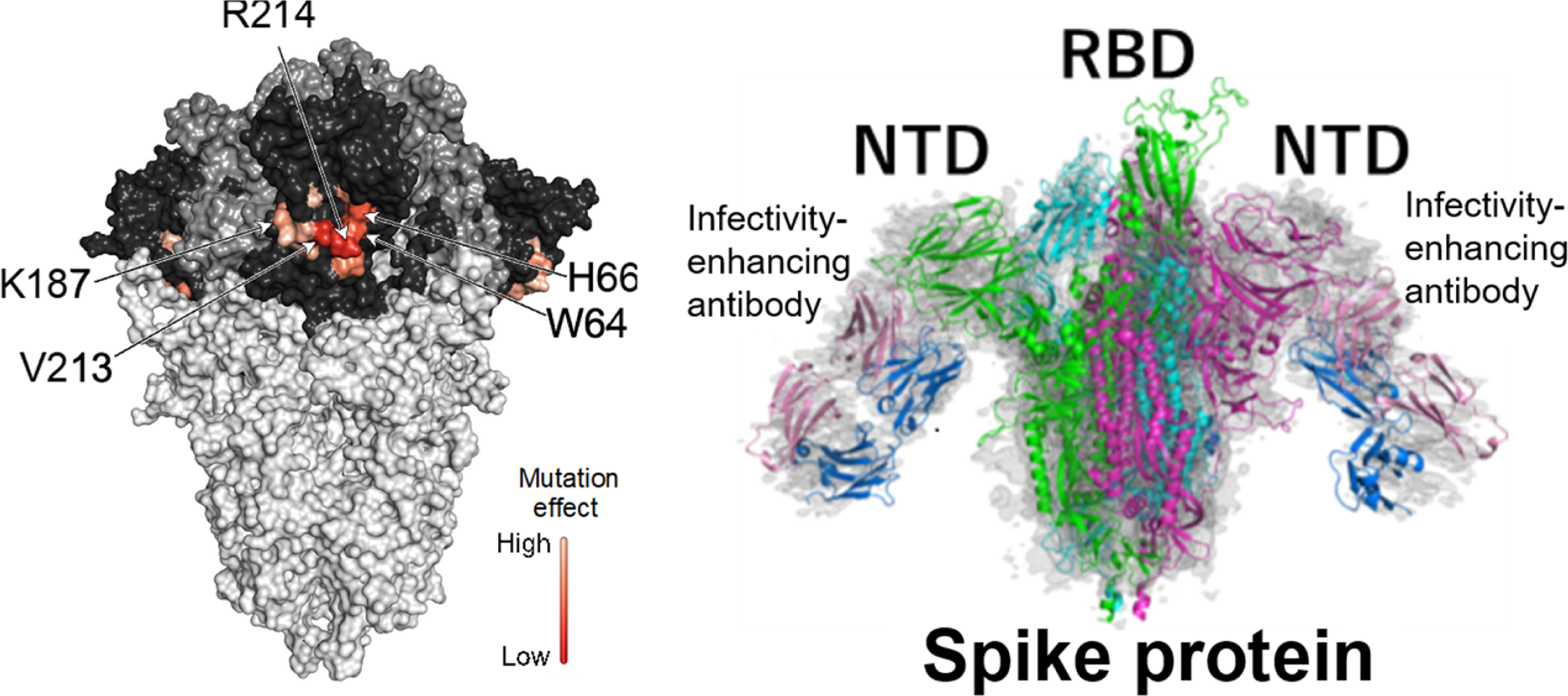
Recognition mechanism of infectivity-enhancing antibodies. Infectivity-enhancing antibodies recognize specific sites in the NTD of the spike proteins. By replacing H66, W64, K187, V213, and R214 with alanine, it was found that all infectivity-enhancing antibodies became unrecognizable (left). Analysis of the binding of the infectivity-enhancing antibodies to the NTD using cryo-electron microscopy revealed that they bound to the lower part of the NTD (right) (modified from Liu et al., Cell 2021 [9]) NTD, N-terminal domain; RBD, receptor-binding domain.
The structural mechanism of infectivity enhancement by anti-NTD infectivity-enhancing antibodies was analyzed. The open conformation of the spike protein RBD facilitates binding of the RBD to the ACE2 receptor, and thus enhances infectivity. Spike proteins are trimeric, and a single molecule of a spike protein contains three RBDs, of which only some form an open conformation. Therefore, the effect of infectivity-enhancing antibodies on open/close conformation was analyzed using an antibody, H014, which specifically recognizes the open RBD. It was found that H014 antibodies bound to the RBD when infectivity-enhancing antibodies bound to the infection-enhancing site of the NTD. On the other hand, antibodies recognizing another site of the NTD did not allow H014 to recognize the RBD. Analysis of Fab and F(ab´)2 fragments of the high-affinity infectivity-enhancing antibodies showed that only F(ab´)2 induced the open RBD to enhance infection, despite both binding to spike proteins to a similar extent. Since the spike proteins are highly mobile molecules on the membrane, when the antibodies bind to the NTD, two NTDs of two neighboring trimeric spike proteins are cross-linked with antibodies and the NTDs are pulled with the movement of the spike proteins. This result proved that the RBD might adopt an open conformation and become highly infective (Fig. 2). Thus, this reveals that the NTD of spike proteins is a key functional domain controlling the RBD function.

Mechanism of action of infectivity-enhancing antibodies. Although the Fab fragments of the infectivity-enhancing antibody bind to the spike protein as conventional antibodies, no infectivity-enhancing effect is observed. On the other hand, because the F(ab´)2 fragments exhibit an infectivity-enhancing effect, an entirely new function was revealed. Herein, the antibody-cross-linked NTDs induce open RBDs by being pulled, resulting in enhanced infectivity (modified from Liu et al., Cell 2021 [9]). NTD, N-terminal domain; RBD, receptor-binding domain; ACE2, angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Ab, antibody.
The infectivity-enhancing antibodies in COVID-19 patients were analyzed. A competitive inhibition method was developed in which the inhibition of the fluorescent-labeled infectivity-enhancing antibody binding to the spike protein by serum-enhancing antibodies was measured. Similarly, a measurement method for neutralizing antibodies against the RBD was developed. Using these methods, infectivity-enhancing antibodies and neutralizing antibodies were measured in COVID-19 patients, and the differences were analyzed. The results showed that infectivity-enhancing antibodies tended to be at high levels in critically ill patients. It was also found that there were a small number of non-infected people with infectivity-enhancing antibodies. Therefore, it is considered that the production of infectivity-enhancing antibodies may be increased if a person with these antibodies is infected with SARS-CoV-2.
This study revealed that when an infectivity-enhancing antibody binds to a specific site of the spike protein of SARS-CoV-2, the structure of the spike protein alters and the infectivity of SARS-CoV-2 enhances. The production of infectivity-enhancing antibodies tends to be higher in critically ill patients, and the production of infectivity-enhancing antibodies could be associated with the severity of the patient’s condition. However, it is still unclear whether infectivity-enhancing antibodies are involved in the exacerbation of infection in the body, and further detailed analysis is required. In addition, it was clarified that the NTD, whose function was previously unknown, is an important region that controls the function of the spike protein. In fact, many recent variants present mutations of the NTD that may affect the function of the RBD. Therefore, the development of infection control methods targeting the NTD is important in the future. Some recent variants have mutations of the recognition sites of the neutralizing antibodies, and these mutations prevent them from acting effectively. Therefore, it is possible that the aggravating effect of the infectivity-enhancing antibodies is higher than the protective effect of the neutralizing antibodies. Further studies are required to clarify the in vivo functions of infectivity-enhancing antibodies and their effects on variants.
Osaka University and HuLA immune Inc. have filed a patent application on the method to detect the enhancing antibodies and the design of spike proteins that do not induce the enhancing antibodies. YL and HA are listed as inventors. YN is an employee of HuLA immune Inc. HA is a stockholder of HuLA immune Inc.
This work was supported by the Japan Agency for Medical Research and Development (AMED) under grant numbers JP19fk0108161 (HA), JP20fk0108542 (HA), and 20fk0108403h (YN, HA) and by JSPS KAKENHI under grant number JP18H05279 (HA).