2023 Volume 5 Issue 1 Pages 23-27
2023 Volume 5 Issue 1 Pages 23-27
Pulse oximetry is a noninvasive technique for the continuous monitoring of arterial oxygen saturation using an oximeter, a standard medical device used for assessing respiratory function. The development of pulse oximeters for the production of generic medical devices is expected to continue. Clinical trials to evaluate oxygen saturation measurements are necessary for the manufacture and commercialization of pulse oximeters. However, no standardized guidelines or methodologies exist for conducting such clinical trials. Therefore, we exploratively aimed to create a hypoxic state in humans to conduct a clinical trial for generic pulse oximeters using a gas-mixing device.
This study aimed to create a convenient in vivo hypoxic environment for pulse oximeter evaluation. Conventionally, a hypoxic chamber is required; however, hypoxia can also be created by inhaling a mixture of nitrogen and oxygen, allowing safe clinical trials to be conducted. This study shows the possibility of evaluating pulse oximeters without special hypoxic chambers, and the findings may be useful for the development of future generic pulse oximeter products.
Pulse oximetry is a noninvasive technique for the continuous monitoring of oxygen saturation in arterial blood and used to detect low oxygen levels in patients [1,2,3]. Arterial blood oxygen saturation is expressed as the percentage of oxygen in the blood (SpO2) when measured with a pulse oximeter or as arterial oxygen saturation (SaO2) when measured directly using a CO-oximeter. Medical professionals determine SpO2 levels periodically and use these as indicators of blood oxygen saturation; hence, the importance of these measurements in detecting hypoxemia. Pulse oximetry relies on detecting differences in the absorption of particular wavelengths of light by oxygenated and reduced hemoglobin. The principle of the pulse oximeter is that oxygenated (oxyhemoglobin) and deoxygenated blood absorb two different wavelengths, 660 (red) and 940 nm (infrared), respectively, from the skin, as measured using the spectrophotometric method. SpO2 is determined by capturing the changes in light absorption caused by reduced hemoglobin [4, 5].
Currently, pulse oximeters are widely used in medical settings around the world and are expected to continue to be developed by medical device companies in the future. According to the “Act on Securing Quality, Effectiveness, and Safety of Pharmaceuticals and Medical Devices (Pharmaceuticals and Medical Devices Act)” enacted in November 2014, the manufacture and sale of medical devices are regulated owing to the postulated risk they might pose to human health [6]. Medical devices in Japan are classified into Classes I to IV according to the “Pharmaceutical and Medical Device Act” of the Ministry of Health, Labor and Welfare. A pulse oximeter is classified as a “managed medical device” or Class II, as it poses a relatively low risk to the human body, even if a defect occurs. The manufacture and sale of pulse oximeters require certification by a third-party certification body designated by the government as a “designated medical device manager”. In principle, Japanese Industrial Standards (JIS) are stipulated as technical standards for the composition of certification guidelines for medical devices.
In 2011, the international standard ISO 80601-2-61 was established for the safety and basic operation of pulse oximeters. Following this, the JIS T 80601-2-61 “Medical Electrical Equipment-Part 2-61: Basic Safety of Pulse Oximeter” was adopted without amending any technical content or configuration based on the ISO 80601–2-61 guidance [7]. In addition, the “Individual Requirements for Basic Performance” standard was published by the Japanese Standards Association [7]. This standard was established by the Ministry of Health, Labor and Welfare and Ministry of Economy, Trade and Industry. According to this standard, the measured values of SpO2 and SaO2 should be compared with the SpO2 accuracy measurement range (for example, 70% to 100% SaO2) as specified by the pulse oximeter manufacturer [7]. Therefore, reducing the available concentration of the fraction of inspired oxygen (FiO2) is necessary in subjects during pulse oximetry clinical trials. Hypoxic chambers, exercise, and measurements of SpO2 at high altitudes can be considered to lower FiO2; however, creating a hypoxic chamber and measuring blood oxygen levels at high altitudes are more expensive than exercise. Nevertheless, maintaining steady-state saturation for a certain period during or after exercise is not always feasible; therefore, we aimed to create a hypoxic environment for the development of a pulse oximeter using a technique that alters the FiO2 by supplying a medical oxygen–nitrogen gas mixture using a gas mixer.
This exploratory study investigated whether a simple method could be used to create a hypoxic environment to develop and validate a pulse oximeter. Therefore, the number of subjects was determined based on feasibility rather than by testing the statistical hypotheses. Healthy volunteers were enrolled in this study, observed during the use of an oximeter, and the obtained data were analyzed.
This study was conducted on healthy volunteers. The inclusion criteria were as follows: (1) age ≥20 but <45 years, (2) a body mass index (BMI) between 18.5 and 30.0 kg/m2, and (3) provided written informed consent to participate in the study. The exclusion criteria were as follows: (1) SpO2 levels <94% in an indoor atmosphere; (2) respiratory diseases, such as chronic obstructive pulmonary disease, asthma, pneumonia, or lung cancer; (3) a history of lung resection; (4) morphological abnormalities of the second and third fingers on both hands and thus unable to obtain SpO2 measurements; (5) methemoglobinemia; (6) diagnosis of sleep apnea syndrome; and (7) patients considered inappropriate for the study by the principal investigator. The study was approved by the Intervention Clinical Research Review Board (approval number: B18-008) of Oita University Hospital in February 2019 and complied with the Declaration of Helsinki and Japanese Ethical Guidelines for Human Medical Research. Written informed consent was obtained from all volunteers prior to trial initiation. The study was registered in the Clinical Trial Registry (UMIN-CTR) in March 2019 (UMIN000036103). Furthermore, we obtained clinical research insurance as a compensation measure for any unforeseen harm or negative health outcomes that could befall any volunteer.
Creating a hypoxic environmentEach healthy volunteer rested in a supine position in the trial room. PULSOX-1® (Konica Minolta Inc., Tokyo, Japan) was used as the standard medical device in this study [8]. First, SpO2 in an indoor atmosphere was determined using a pulse oximeter. Next, blood pressure and pulse rate were measured. Subsequently, trial SpO2 readings were determined after confirming no change in the numerical values of the readings from the aforementioned measurements. The volunteers inhaled medical oxygen–nitrogen gas from a gas mixer (KOFLOC Corp., Kyoto, Japan) through a gas inhalation mask (Koken Co., Ltd., Tokyo, Japan), as illustrated in Fig. 1, while covering their nose and mouth.
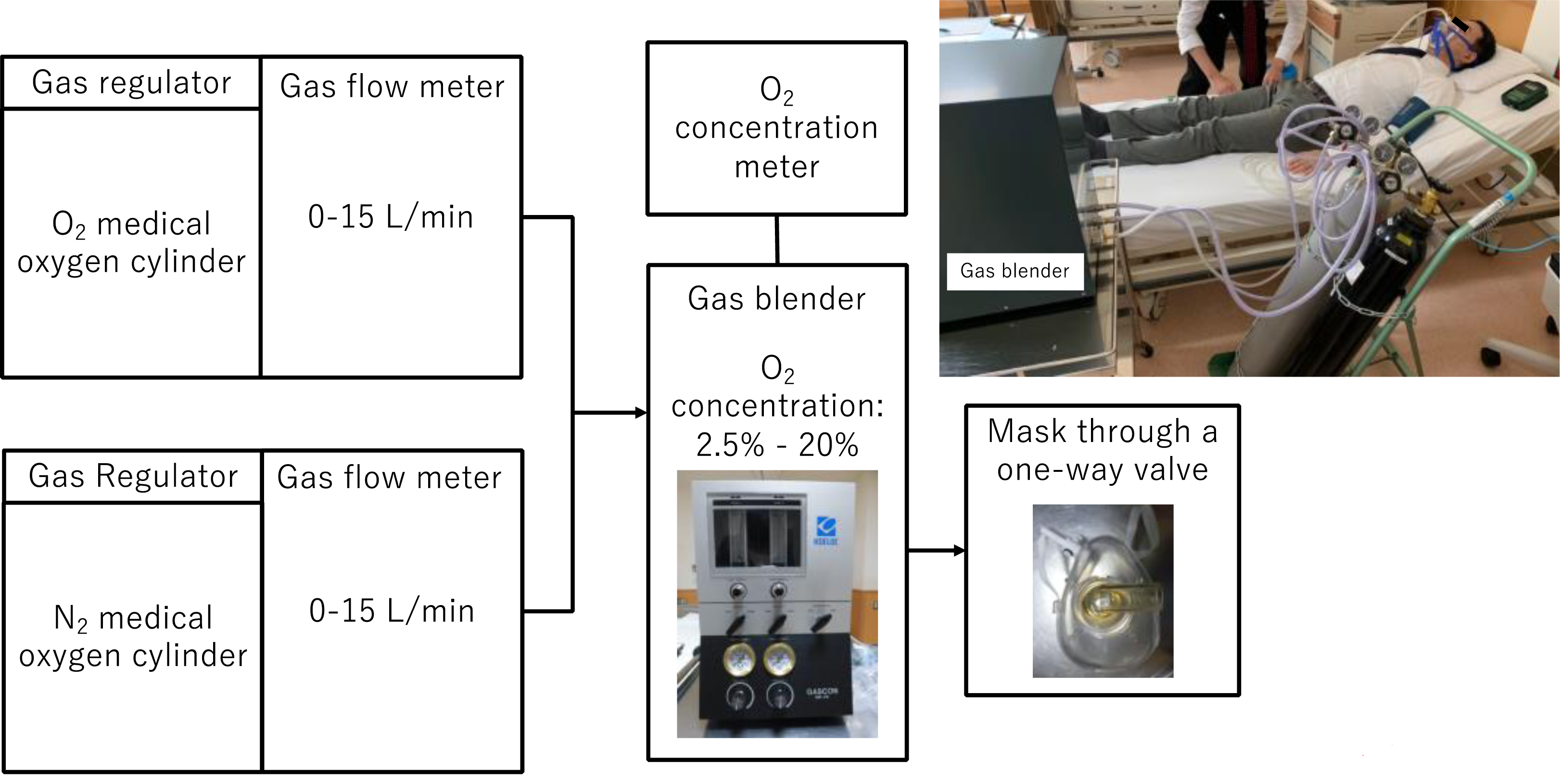
Overview of the device used to create hypoxic conditions.
The oxygen intake concentration was adjusted to 2.5%, 3%, 4%, 10%, and 20% by regulating the flow rates of oxygen and nitrogen gas and continuously monitored using an oxygen monitor inserted on the intake side. Volunteers inhaled medical oxygen and nitrogen gas while inspiratory oxygen was kept constant; after 5 min, we confirmed that the value measured using the pulse oximeter reached a stable level before recording the reading. SpO2 measurements were repeated every 30 sec, with a total of 10 measurements performed, as depicted by the trial flow chart in Fig. 2
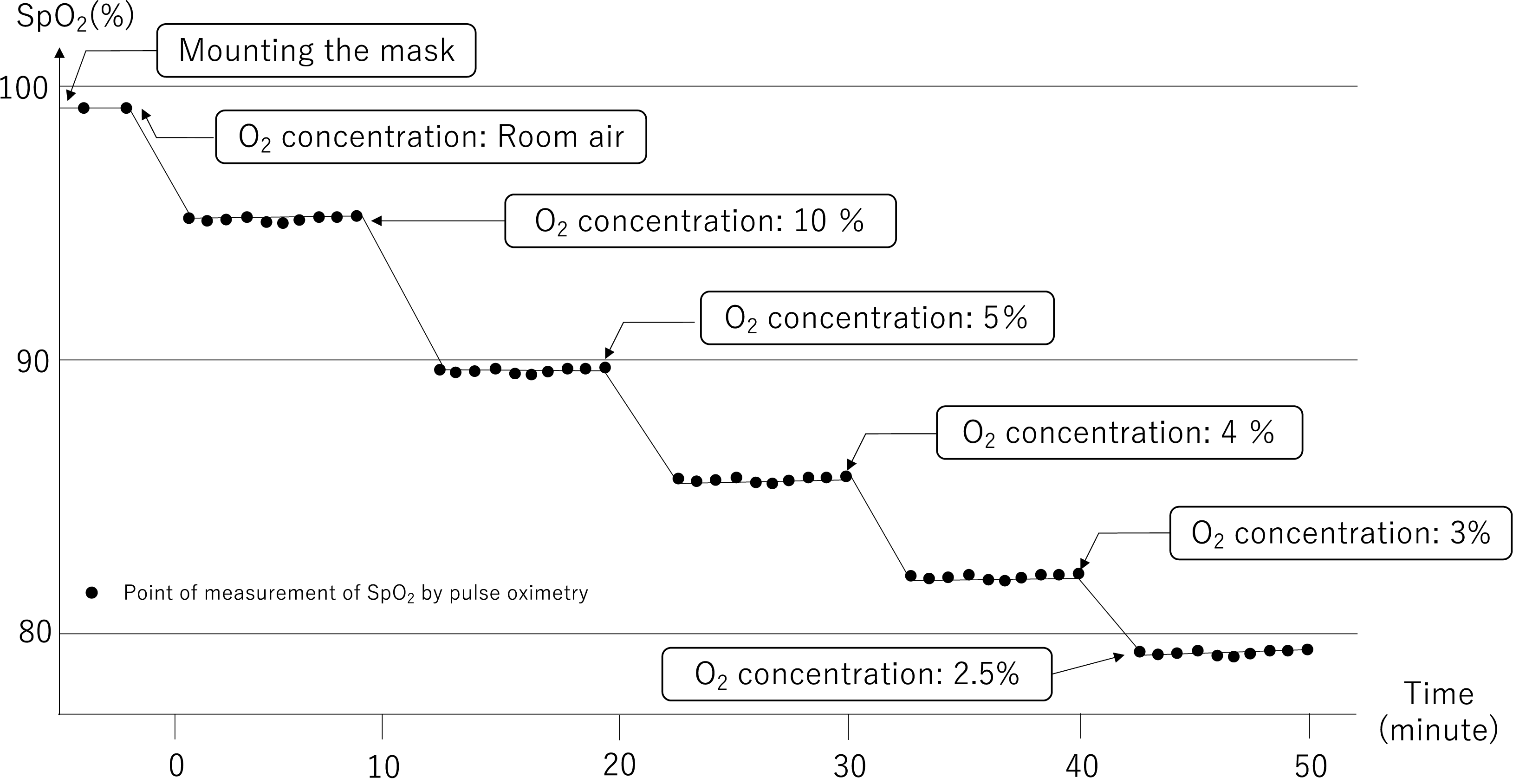
Trial flowchart depicting inhaled gases and subsequent measurements.
Blood pressure, pulse, and consciousness levels were determined to evaluate safety. The volunteer was moved to the next hypoxic stage if the decrease in SpO2 was <2% of the previous step when the pulse oximeter reached a stable level after 5 min. If SpO2 became 75% or less, subsequent measurements were not performed for safety reasons.
Six healthy volunteers were screened, and one was excluded because the BMI was outside the reference range. Five volunteers (four males and one female) who were finally included in the study inhaled oxygen–nitrogen gas to create a hypoxic environment. One volunteer experienced severe pain from the base to the bridge of the nose while wearing the mask, and the process was discontinued before a hypoxic environment was attained. Another volunteer gradually shifted to a hypoxic state; however, no decrease in oxygen saturation was observed. Furthermore, the SpO2 of this volunteer was 97% even after stopping oxygen gas and inhaling only nitrogen gas. Thus, these two cases were excluded from data analysis. The median age of the subjects enrolled in the analysis was 35 years (range: 30–36 years) (Table 1). Regarding safety concerns, nose pain while wearing a mask was an adverse event caused by the experimental conditions. No other adverse events, such as impaired consciousness, abnormal blood pressure and pulse, dyspnea, or other respiratory symptoms, were observed. Table 2 shows the average SpO2 values of all the healthy volunteers. A decrease in SpO2 was observed as oxygen concentration decreased, although individual differences exist.
| Volunteer | Ethnicity | Sex | Age (years) | Height (cm) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|---|---|
| Volunteer #1 | Japanese | F | 36 | 159 | 60 | 24 |
| Volunteer #2 | Japanese | M | 35 | 171 | 69 | 24 |
| Volunteer #3 | Japanese | M | 24 | 174 | 66 | 22 |
BMI: body mass index; F: female; M: male.
| Oxygen concentration | Room air | 10% | 4% | 3% | 2.5% |
|---|---|---|---|---|---|
| Mean (%) | 97.7 | 95.1 | 90.9 | 89.8 | 86.8 |
| SE | 1.25 | 2.70 | 1.64 | 4.23 | 3.63 |
SE: standard error.
Figure 3 shows the SpO2 readings from each healthy volunteer and changes over time based on inhaled gas as measured by the PULSOX-1®︎ pulse oximeter.
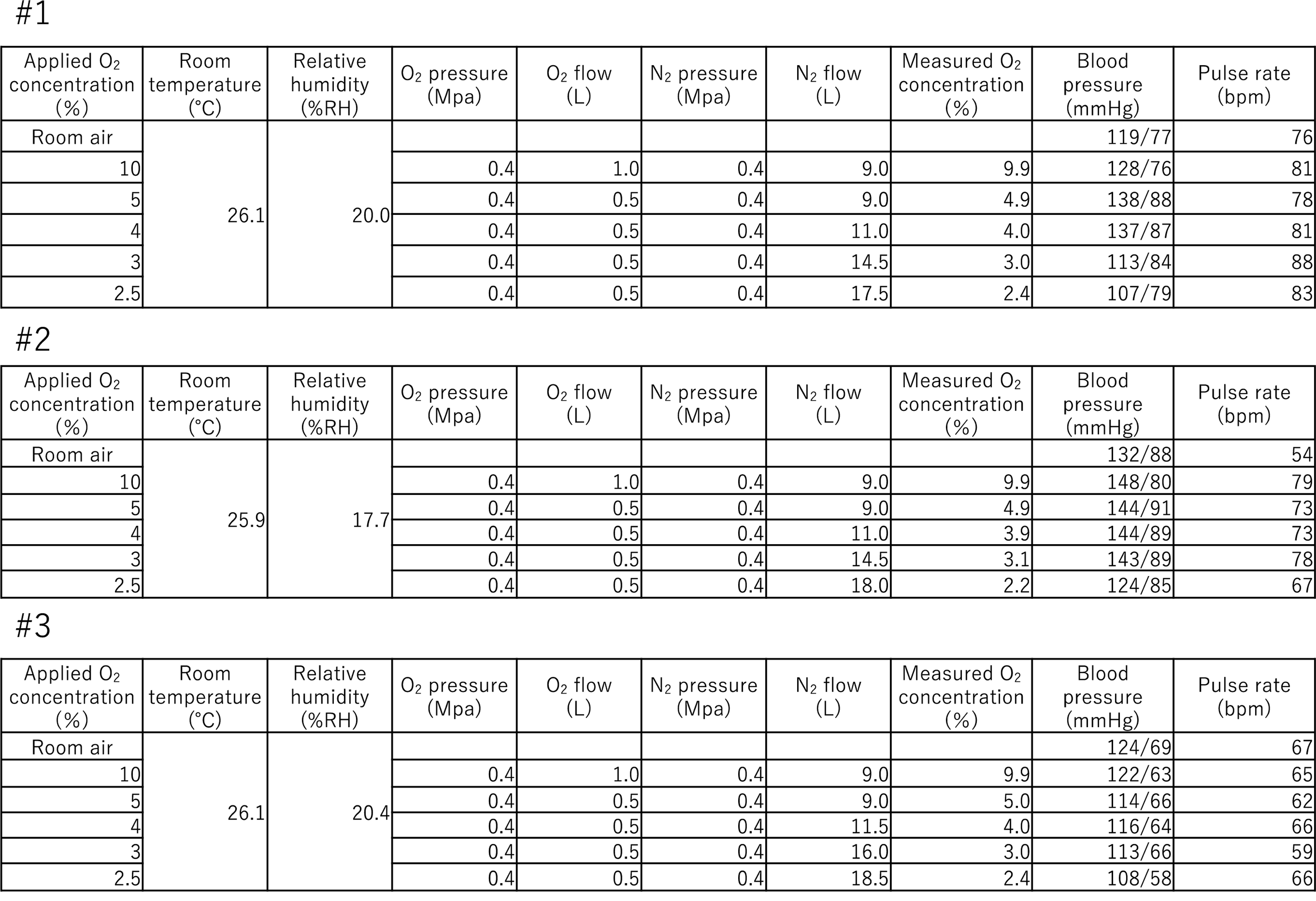
Setting up the environment and equipment during clinical trials and vital signs of each healthy volunteer. This shows the hypoxic setting shown in Fig. 1 and vital signs as a safety assessment for each volunteer. Blood pressure and pulse rate are values at any given time during that hypoxic condition.
In this study, healthy volunteers inhaled a mixture of medical oxygen and nitrogen gas to create a hypoxic state, and their SpO2 values were measured using an existing pulse oximeter in the range of 79–99%, as a standard medical device. The hypoxic condition device (Fig. 1) allowed subjects to inhale gas with a lower oxygen concentration than the room atmosphere, thus lowering their FiO2 values as we were able to create a constant hypoxic environment with SpO2 readings between 79% and 99%. We postulate that this method could be used to develop and validate pulse oximeters as creating a hypoxic environment was possible.
In the JIS T 80601-2-61 “Medical Electrical Equipment-Part 2-61: Individual Requirements for Basic Safety and Basic Performance of Pulse Oximeters”, an invasive low oxygen saturation test for humans is recommended. However, the Medical Device Technology Expert Committee, a subcommittee of the JIS Committee, also accepts noninvasive tests that accurately measure SpO2 using pulse oximetry techniques. The general purpose of the pulse oximeter is to be the “gold standard” for accurately determining SpO2 by comparing its results with blood SaO2 measurements obtained using the CO-oximeter, thus confirming its validity. We further propose the development of generic medical devices using the PULSOX-1® oximeter as the secondary standard.
Six healthy volunteers were screened, and one was excluded because his BMI did not meet the selection criteria. Five healthy volunteers (four men and one woman) were administered inhaled medical oxygen–nitrogen gas. One volunteer dropped out of the study because of nose pain while wearing the mask after adjusting the mask to achieve a perfect fit. The second volunteer had arterial oxygen saturation levels that did not decrease. Subsequent investigations revealed that this volunteer had an exercise habit of running long distances. The ability to take oxygen into the body in 1 min is called maximal oxygen uptake (VO2 max) and is an indicator of an athlete’s performance during marathons [9,10,11,12]. VO2 max increases in athletes, particularly those who run marathons. In contrast, increasing altitude leads to a decrease in maximal oxygen uptake [13, 14]. As VO2 max increases in athletes, arterial oxygen saturation mildly decreases even in low-oxygen environments, such as in the highlands. Therefore, this volunteer was thought to have a high oxygen-carrying capacity similar to that of athletes because of running long distances on a daily basis. It may be necessary to exclude “athletes who routinely perform high-intensity aerobic exercise estimated to cause increased VO2 max or those who perform similar exercises” for studies such as the current one based on this result.
In this clinical study, in which a hypoxic environment was created by the inhalation of a mixture of oxygen and nitrogen, SpO2 values ranged from 79% to 99%, with no lower values obtained. Room air inflow through the gap between the mask and face was possibly the cause, resulting in increased oxygen levels, which is also a limitation of this study. Conversely, if the mask is too firmly attached to the face, it may not be able to withstand long-term use. This technique may be difficult to apply in cases in which a more hypoxic environment is required. Future risk assessments are required to address arrhythmias due to hypoxia exposure, long-term effects, and impact of repeated testing, although no serious adverse events occurred in this clinical study.
LimitationsThe results could not be statistically examined owing to the exploratory nature of this study. Nonetheless, setting the number of subjects and planning a clinical trial was possible based on the data obtained. In addition, the SpO2 values at each oxygen concentration could not be predicted, although individual differences were substantial and a hypoxic environment could be created. These individual differences might be caused by the adhesion strength when wearing a gas mask. The indoor air seeped in, and the oxygen concentration increased when a gap was created between the mask and the face. One of the issues to be addressed is the balance between the increased adhesion of the mask for accuracy and load on the participants for safety.
Writing—original draft preparation, N.O.; writing—review and editing, H.W., K.S., K.H., Y.N., M.K., I.O., H.K., H.I., and N.U. All authors have read and agreed to the published version of the manuscript.
This study was a joint project with HOKS Co., Ltd. As a “2018 Medical-Engineering Collaboration Research and Development Promotion Subsidy Project (Oita Prefecture Medical Robot and Equipment Industry Council)”, this work was supported by HOKS Co., Ltd., which added subsidy expenses from Oita Prefecture. HOKS Co., Ltd. was involved in the study design and writing of the report; however, it was not involved in data collection or the decision to submit the article for publication. The Oita Prefecture had no involvement in this study.
The authors have no conflicts of interest to declare.
We would like to thank HOKS Co., Ltd., Eto Oxygen Co., Ltd., and the staff of the Clinical Biomedical Engineering Center of the Oita University School of Medicine for their support.