2024 Volume 6 Issue 1 Pages 28-36
2024 Volume 6 Issue 1 Pages 28-36
Macrophages infiltrate lesions in diseases such as inflammation, tumors, and fibrotic tissues. Once recruited to the lesion sites, macrophages are activated and acquire diverse phenotypes depending on the stimuli in the microenvironment. Activated macrophages contribute to disease progression and are crucial targets in disease therapy because of their significant impact on immune responses through various effector functions, such as cytokine production, phagocytosis, and antigen presentation. Various approaches, including the regulation of macrophage recruitment, activation, and cancer suppression, have been demonstrated to have therapeutic effects. The challenge in modulating macrophages is that therapies aimed at regulating one aspect of macrophage properties may have unintended effects on another, potentially causing adverse effects. This review describes a therapeutic approach for regulating macrophages, focusing on FROUNT, a novel target molecule that induces macrophage migration and activation of regulatory molecules. FROUNT promotes signaling via chemokine receptors CCR2 and CCR5, modulating the intensity of chemokine signaling responses; thus, becoming a promising target for modulating macrophage function and safely controlling cancer and inflammation. By disrupting the interaction between FROUNT and chemokine receptors, we selectively regulated chemokine receptor signals mediated by FROUNT, suppressed the infiltration and activation of macrophages at lesion sites, and mitigated the pathogenesis of macrophage-related conditions such as nephritis and cancer. Targeting molecules that promote chemokine receptor–signaling could be a next-generation macrophage-targeted therapy with fewer side effects and higher efficacy than existing drugs for cancer and inflammation.
Macrophages are involved in various diseases; however, owing to their diversity, regulating one aspect of macrophage properties may cause unintended adverse effects. We identified the region of chemokine receptors that FROUNT, a chemokine receptor-associated molecule that promotes macrophage migration and activation, binds to; and determined that diseases such as cancer and nephritis can be controlled by inhibiting the interaction between chemokine receptors and FROUNT. We propose a next-generation macrophage-targeted therapy that safely controls cancer and inflammation by regulating FROUNT.
Macrophages infiltrate numerous lesion sites in various diseases, such as cancer, inflammation, and fibrosis, and significantly affect inflammatory and immune responses. Depending on the tissue microenvironment, macrophages are activated and polarized with different properties, exhibiting characteristic cytokine and chemokine production, phagocytosis, and antigen-presenting abilities [1]. In an inflammatory setting, in the presence of pathogen-associated or danger signals derived from damaged tissues, blood monocytes are recruited to the inflammation site and differentiate into proinflammatory macrophages that produce inflammatory cytokines and chemokines with antimicrobial or phagocytic activities [2]. Macrophages also participate in the resolution phase and suppress inflammation by secreting anti-inflammatory cytokines, such as TGF-β and interleukin (IL)-10, and matrix metalloproteinases involved in tissue remodeling [3]. This type of macrophage is also present in tumors and contributes to tumor growth. In vitro studies have demonstrated that macrophages can differentiate into two main types: classical inflammatory (also known as M1 type) and anti-inflammatory (referred to as M2 type) [4]. However, in vivo studies and disease contexts have revealed a more complex and overlapping spectrum of macrophage functions and properties that extend beyond this binary classification [5]. Macrophages play a critical role in both innate and adaptive immune responses owing to their substantial cytokine production capacity and relatively long lifespan. However, should they act excessively and protractedly, it can cause disease. Based on the evidence of their accumulation in various diseases and the therapeutic effects obtained by removing or inhibiting macrophages in animal experiments [6,7,8,9], macrophage targeting is expected to enable control of various diseases (Fig. 1). However, the diverse properties of macrophages, such as their ability to suppress immunity in cancer and promote or resolve inflammation and immune responses depending on the disease stage, increases the challenge of controlling macrophages without affecting their essential physiological functions, which can cause adverse side effects. Herein, we introduced the possibility of a therapeutic approach to regulate macrophages, focusing on FROUNT, a macrophage migration and activation-regulatory molecule.

Clinical trials assessing changes in macrophages as an outcome measure (188 trials) were categorized based on the diseases they target. Results were sourced from ClinicalTrials.gov, using the search term “macrophage” and field “outcome measure” as of February 2024. COPD: chronic obstructive pulmonary disease; IPF: idiopathic pulmonary fibrosis.
Various approaches are underway to develop macrophage-regulating agents. Additionally, existing drugs have the potential to modulate macrophage function and can be classified into four categories based on their mechanism of action: blocking recruitment, blocking activation, promoting activation, and suppressing release (Fig. 2). Several approaches have opposite effects depending on the disease situation. For example, immune responses activated during cancer therapy but suppressed during inflammatory diseases such as autoimmune diseases [10, 11].

Therapeutic agents, currently in use or under development, that can affect macrophages are categorized into four groups based on their mechanisms of action: blocking recruitment, blocking activation, promoting activation, and releasing suppression.
Blood monocytes are the major source of macrophages that accumulate at lesion sites [12,13,14] and are recruited in response to chemoattractants such as chemokines, cytokines, and complements produced at the lesion sites. Therefore, therapeutic approaches targeting chemoattractants or their receptors have been investigated in research and clinical trials for the treatment of cancer and inflammatory diseases [9, 15, 16]. C-C motif chemokine receptor 2 (CCR2) is a major regulator of monocyte migration [12, 17], and its therapeutic effects have been investigated. Monocytes and macrophages also utilize the CCR5 for their migration, especially within tissue [18]. Dual inhibitors of CCR2 and CCR5 have been developed and their therapeutic effects have been investigated in nonalcoholic steatohepatitis and cardiovascular disease [19, 20]. Colony stimulating factor 1 (CSF1), a crucial cytokine for macrophage maintenance, is a chemoattractant for monocytes and macrophages. Anticancer effects have been reported through CSF1 receptor inhibition [21, 22].
Blocking activationPrimarily aimed at treating inflammatory diseases, various drugs have been developed or used to inhibit macrophage activation. This involves using classical steroids that inhibit inflammatory cytokines and adhesion molecules at the transcriptional level [23]. Recently, Janus kinase (JAK) inhibitors have been used to treat autoimmune diseases such as rheumatoid arthritis [24]. These inhibitors act on various cytokine receptors involved in macrophage activation and polarization, such as IFNγ- and IL-4, and are therefore expected to act on macrophages, especially in their polarized state [25, 26]. Syk tyrosine kinase, which is required for B-cell antigen receptor activation [27], is also involved in Fc receptor signaling in macrophages [28]; and Syk inhibitors were approved for treating idiopathic thrombocytopenic purpura by macrophage phagocytosis inhibition [29]. Neutralizing antibodies or receptor antagonists against the inflammatory cytokines, Tumor necrosis factor (TNF) and IL-6, produced by macrophages leading to their activation, are also effective against rheumatoid arthritis and inflammatory bowel diseases, partly because of their reprogramming of macrophages into an anti-inflammatory phenotype [30,31,32]. However, this approach carried the risk of reactivating controlled infections such as tuberculosis [33]. Blocking IL-1β, another key inflammatory cytokine, has dual effects: it suppresses inflammation in autoimmune diseases and exerts antitumor effects by inhibiting the recruitment of myeloid-derived suppressor cells [34]. IL-1β is cleaved from pro-IL-1β by caspase-1, which is activated by inflammasome activation. Recently, inhibitors targeting the canonical inflammasome NLRP3 were assessed, demonstrating their reduction of atherosclerotic lesions [35].
Promoting activationAgents that activate macrophages are under development for cancer treatment, including CD40-agonist antibodies that mimic activation signals from T cells. Several clinical trials have been conducted [36]. However, adverse effects, including hepatotoxicity, cytokine release syndrome and thrombocytopenia, which is attributed to macrophage activation, have been identified [37]. Various toll-like receptor agonists and agonists for abnormal double-stranded DNA sensor stimulator of interferon genes (STING) have been explored for their adjuvant properties, aiming to enhance the induction of immune responses, thereby potentially improving the effectiveness of vaccines and immunotherapies [38]. Recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) agents, used to stimulate macrophage differentiation and mobilization, are currently applied in clinical settings. Inhaled GM-CSF has been used to treat pulmonary alveolar proteinosis, which is caused by autoantibodies against GM-CSF, and has shown therapeutic efficacy through macrophage transfer [39, 40].
Releasing suppressionIn cancer, macrophages acquire immunosuppressive properties through signals from scavenger receptors [41]. These signals induce suppressive properties in macrophages that, normally, inhibit macrophage activation and terminate their inflammatory response during the resolution phase of inflammation. Therefore, agents that inhibit these scavenger receptors are currently being investigated [42]. High expression of MARCO, a macrophage receptor with a collagenous structure, is correlated with poor prognosis in many cancers [43]. Inhibiting CD47 and its receptor, SIRPα expressed on macrophages to mediate “don’t eat me” signals, signals that inhibit macrophage phagocytosis. Furthermore, they are being developed to facilitate phagocytosis of tumor cells that acquire high CD47 expression [44]. Attempts have been made to increase the efficiency of B cell lymphoma depletion by fusing proteins that combine an antibody against the B cell marker CD20 with the CD47-binding site of SIRPα [45]. Currently, CD24 and Siglec-10 having been identified as another “don’t eat me” signal [46], are being evaluated using blocking antibodies for their pathway [47]. The leukocyte immunoglobulin-like receptor B (LILRB) family has attracted attention as a novel target for cancer immunotherapy. LILRB1 and LILRB2, which are expressed on macrophages, bound to MHC class I molecules in tumor cells, mediate inhibitory signals via their immunoreceptor tyrosine-based inhibitory motif [48]. Currently, blocking antibodies against these receptors is being investigated for anticancer efficacy [49].
To overcome the difficulty of developing macrophage regulators that require different controls for different diseases, we focused on chemokine receptor signaling regulators, which mediate cellular migration and activation, as an effective way to regulate macrophages. We searched for molecules that bind to CCR2, which is expressed on monocytes and macrophages, and is responsible for the migration and activation of inflammatory sites. Molecules binding to the C-terminal region of CCR2 were screened from a cDNA library of THP-1 cells using the yeast two-hybrid method. Terashima et al. identified several candidate molecules, one of which is FRONT, that efficiently induced a migratory response by promoting the Rac/PI3K/lamellipodium cascade in cells [50].
Receptors regulated by FROUNTTo date, approximately 50 chemokines and 20 chemokine receptors have been identified. Distinct types of immune cells and their states of immune cells express different sets of chemokine receptors that spatiotemporally regulate immune cell dynamics. The correspondence between chemokines and chemokine receptors is characterized by redundancy. A single chemokine can bind to multiple chemokine receptors, and conversely, a single chemokine receptor can bind to various types of chemokines [51]. This complexity in the regulation of the chemokine system has made the development of therapeutic agents targeting chemokines and chemokine receptors challenging. Targeting intracellular regulators of chemokine receptors may contribute to the comprehensive regulation of multiple complementarily functioning receptors. Notably, FROUNT binds to CCR5 in addition to CCR2 [52], and CCR2 and CCR5 are required to mobilize monocytes and macrophages into tissues; additionally, they are known for their contributions in diseases, such as cancer [53,54,55,56], autoimmune diseases [57,58,59], and fibrosis [8, 60, 61].
FROUNT-GFP reporter mice were generated by inserting a GFP gene downstream of the FROUNT promoter, allowing the in vivo quantification of FROUNT expression [62]. FROUNT is highly expressed in monocytes in the blood and in tumor-accumulated macrophages. In an analysis of FROUNT mRNA expression in surgical specimens from patients with lung adenocarcinoma conducted in collaboration with the Chiba Cancer Center, patients with high FROUNT expression had poor postoperative prognosis. Therefore, FROUNT is expected to be a molecule that promotes cancer and a potential target for cancer therapies. In a microarray analysis of prostate cancer, FROUNT, also called pericentrin 1, was found to be significantly upregulated in metastatic or advanced prostate cancer [63]. FROUNT is also involved in the mobilization of stem cells, suggesting a role for FROUNT beyond immune cells [64].
Screening for FROUNT inhibitorsWe screened for FROUNT inhibitors to develop therapeutics for cancer and inflammatory diseases involving CCR2 and CCR5, on which FROUNT acts. Since chemokine receptor–signaling is also responsible for various physiologically essential responses, we screened compounds that inhibit the interaction between FROUNT and chemokine receptors to selectively control disease-related responses involving FROUNT. To achieve this, a 16-amino acid sequence in the membrane-proximal C-terminal region of CCR2 was identified as the minimal-binding region for FROUNT [65], and synthetic peptides of the corresponding sequence were used for high-throughput screening of FROUNT-CCR2 inhibitors. From a library of >132,000 compounds provided by the Drug Discovery Research Institute at the University of Tokyo, seven hit compounds were obtained through multi-step screening via the inhibitory activity of the FROUNT-CCR2 interaction, binding activity to FROUNT, chemotaxis inhibitory activity independent of cytotoxicity, and migration inhibitory activity in vivo (Fig. 3). The cancer-inhibitory effects of candidate compounds were also assessed. The library included existing drugs, among which disulfiram exhibited potent inhibitory activity against FROUNT [62]. Disulfiram is a safe drug that has been used clinically for >70 years for treating alcohol dependency owing to its aldehyde dehydrogenase (ALDH) inhibitory activity [66]. Consequently, we evaluated the FROUNT inhibitor disulfiram in various disease models.

A multi-step screening strategy was employed to identify FROUNT inhibitors that target macrophages by inhibiting the interactions between FROUNT and CCR2/CCR5. We used the homogeneous time-resolved fluorescence (HTRF) assay to screen for compounds that selectively inhibit the interaction between FROUNT and CCR2. This approach enabled us to discover inhibitors that specifically block the FROUNT-mediated signaling pathway within the overall chemokine receptor signaling.
The effects of targeting FROUNT on cancer progression were examined in FROUNT-deficient mice. Tumor cells transplanted into FROUNT-deficient mice showed markedly reduced growth compared to that in the control. The number of infiltrating macrophages in the tumor tissues was also reduced. Similarly, disulfiram, identified as a FROUNT inhibitor in our screening, showed a tumor-suppressive effect when administered four days after the transplantation of Lewis lung carcinoma (LLC) or B16 melanoma tumor cells [62]. Furthermore, combined treatment with disulfiram and the immune checkpoint inhibitor programmed death-1 (PD-1) antibody almost completely suppressed LLC tumor growth. Additionally, even in B16 melanoma cells that were unresponsive to the PD-1 antibody alone, the combination of disulfiram and PD-1 exhibited a significant tumor-suppressive effect. A synergistic increase in the number of cytotoxic T cells in the tumor tissue was observed in the combination group [62]. Therefore, because disulfiram with FROUNT inhibitory activity showed a tumor-suppressive effect and demonstrated the usefulness of FROUNT inhibitors in nonclinical settings, a clinical study of disulfiram in combination with nivolumab in patients with unresectable gastric cancer was conducted at the National Cancer Center (jRCTs031180183). In addition to confirming the safety of the combination, single-cell transcriptome analysis of biopsy specimens conducted as an accompanying study revealed changes in macrophage subsets induced by disulfiram administration (unpublished).
FROUNT inhibitor suppresses nephritisIn addition to cancer, we are exploring other inflammatory diseases as potential targets of FROUNT inhibitors. We recently reported the potent inhibitory effect of the FROUNT inhibitor disulfiram on nephritis [67]. Disulfiram markedly suppressed the anti-glomerular basement membrane in a glomerulonephritis rat model, an immune-mediated nephritis where macrophages play an important role in pathogenesis [68]. Disulfiram dramatically reduces monocyte/macrophage accumulation in the glomeruli by inhibiting the chemotactic response of monocytes/macrophages and their activation to produce cytokines and chemokines. We also synthesized disulfiram derivatives with enhanced FROUNT and reduced ALDH inhibitory activity. Among these, DSF-41, which showed a four-fold increase in IC50 for FROUNT inhibitory activity, produced comparable nephritis suppression at one-fifth of the DSF dose (20 mg/kg for DSF-41 vs. 100 mg/kg for DSF) [67]. Disulfiram also suppressed renal fibrotic lesions that occurred after glomerulonephritis. Additionally, we observed antifibrotic effects of disulfiram in mouse models of pulmonary fibrosis. These results indicate that inhibiting monocyte-macrophage chemokine responses may be a promising therapeutic strategy for glomerulonephritis and other diseases with inflammation-induced fibrotic changes. Disulfiram, a clinically safe drug, may serve as a new therapeutic agent for treating glomerulonephritis by modulating the chemokine responses of monocytes and macrophages.
Beyond chemotaxis, new FROUNT functions being discoveredFirst, we focused on the effect of FROUNT as a chemokine receptor–signaling molecule in regulating monocyte/macrophage migration. However, our analysis revealed that FROUNT regulation also altered the characteristics of macrophages present at the lesion site, with decreased expression of CD86 and MHC class II, markers of M1 macrophages, which are inflammatory macrophages, and CD206, a marker of M2 macrophages, which are anti-inflammatory and tumor-promoting macrophages [62]. The expression of inflammatory cytokines and chemokines, such as TNFα and CCL2, was also suppressed in FROUNT-deficient macrophages and macrophages treated with disulfiram [67]. These molecules are induced upon activation, indicating that FROUNT regulates macrophage activation rather than skewing toward M1 or M2 macrophages. We are currently analyzing the detailed molecular mechanisms by which chemokine receptor–signaling molecules regulate the expression of stimulus-dependent cytokines and activation markers.
While various methods to control macrophages that exacerbate various diseases are being explored, targeting FROUNT, a chemokine receptor–signaling regulator, is expected to have the following advantages: (1) may reduce side effects by being a signal-promoting molecule and specifically targeting the interaction between FROUNT and chemokine receptors, without affecting physiologically necessary signals, and selectively regulating signals associated with disease progression; (2) has the potential to simultaneously regulate multiple functionally complementary receptors that share a motif for binding to FROUNT and is expected to have a strong effect; (3) can control the activation of macrophages and, therefore, control the entire disease process by targeting macrophages infiltrating the lesion site and resident macrophage (Fig. 4).
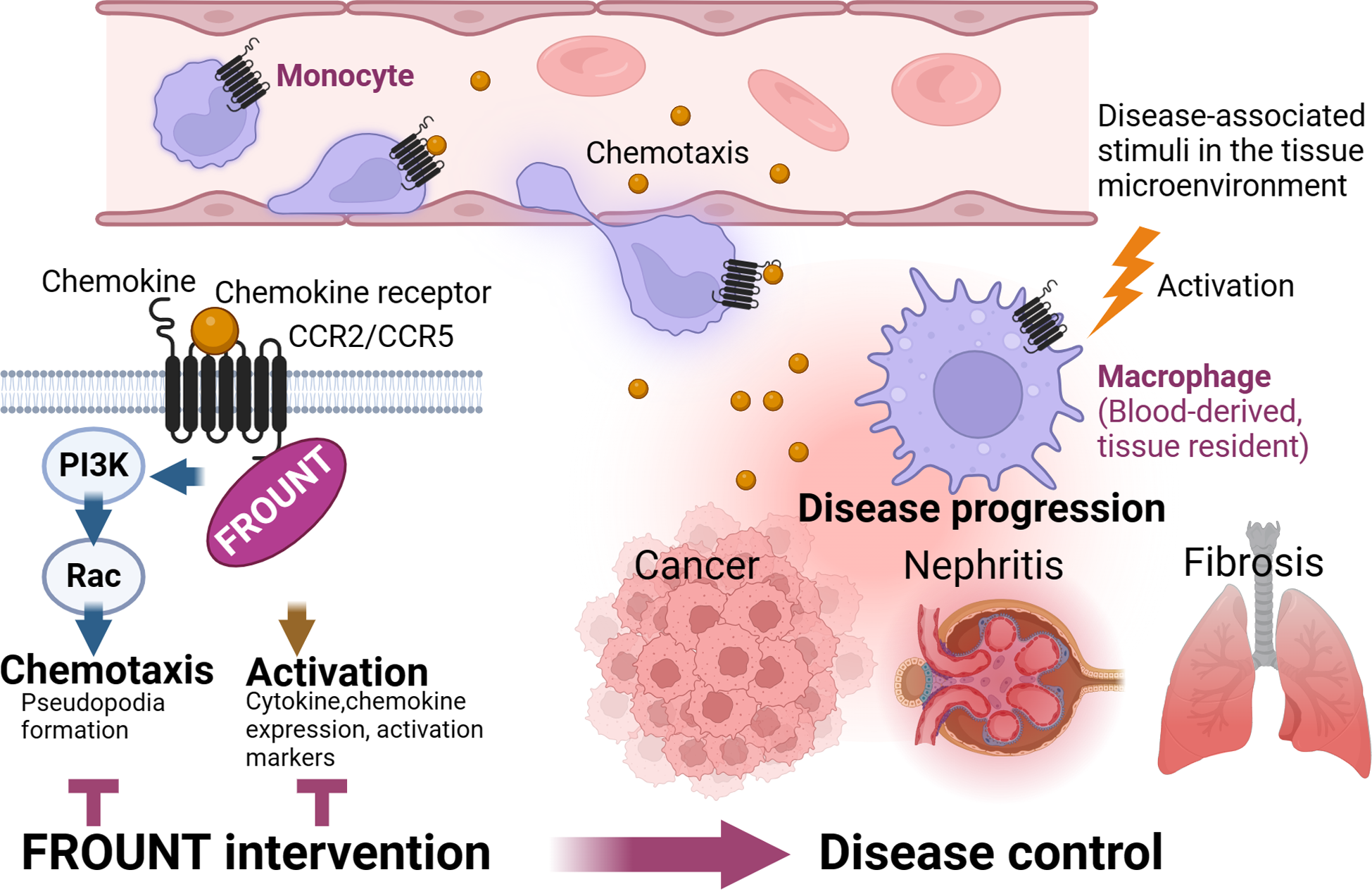
Summary of the advantage of targeting the chemokine signal amplifier FROUNT.
We considered the clinical application of disulfiram, which has been used for a long time, prioritizing its clinical application. However, we are currently studying the improvement of an inhalant formulation to deliver FROUNT inhibitory activity more stably in vivo and are developing a new drug with FROUNT-specific inhibitory activity. We believe that regulating the multifaceted response of macrophages will enable the treatment of various diseases with fewer side effects and we will continue our developmental efforts. Additionally, future therapeutic strategies are expected to target FROUNT-like receptor regulators for other chemokines and G protein-coupled receptors.
The authors declare that they have no conflict of interest to disclose.
This study was supported in part by Practical Research for Innovative Cancer Control, Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development (AMED), JSPS KAKENHI [grant numbers 20K07553 and 21H02755], and Initiative for Realizing Diversity in the Research Environment of MEXT, Japan. Figs. 3 and 4 were created with BioRender.com.