Abstract
In recent years, introducing virtual control groups (VCGs) into toxicology studies is
increasingly discussed because of the 3Rs and non-human primate (NHP) supply issues.
Evaluating toxicology study results using historical control data is not new; however,
introducing a VCG means replacing the concurrent control group in a toxicology study with
a VCG, thereby reducing the number of animals used by approximately 30%. While it may be
possible to conduct a toxicology study of a developmental compound in which the concurrent
control group is replaced with a VCG, the scientific appropriateness of introducing a VCG
and its regulatory acceptability needs to be considered. Therefore, we identified the
following five issues that may arise when implementing a VCG: 1) regulatory requirements,
2) common issues when introducing a VCG, 3) issues related to histopathological
examinations when introducing a VCG, 4) statistical analysis when introducing a VCG, and
5) facility monitor (sentinel) animals. Current regulatory guidelines require a concurrent
control group for a pivotal toxicology study, whose results, if do not meet the
requirements of these guidelines, cannot be used for new drug approval applications. Even
if the use of VCGs is justified from animal welfare and scientific points of view, it is
critical that the industry work with health authorities to ensure that data from these
studies are accepted. The Japan Pharmaceutical Manufacturers Association will continue to
hold necessary discussions with key stakeholders to accelerate efficient and effective new
drug development pertaining to the use of VCGs.
Highlight
The Japan Pharmaceutical Manufacturers Association (JPMA) Taskforce Team has continuously
discussed concerns about introducing virtual control groups (VCGs) into non-clinical
toxicology studies from different perspectives at team meetings. To ensure that the issues
identified in this study ultimately lead to efficient and effective new drug development,
the intent was to expand discussions involving key stakeholders, including regulatory
authorities.
Introduction
Animal studies are a critical component of drug development. However, efforts are being
made to reduce the number of laboratory animals used. Over 50 years ago, the principles of
the 3Rs (Replacement, Reduction, and Refinement) were developed to promote ethical animal
research [1]. In December 2022, a U.S. law was
enacted, which eliminated the requirement that drugs under development be tested in animals
prior to being administered to humans in clinical trials [2]. Although this new law was groundbreaking, it did not ban animal
experimentation. In reality, there is no guarantee that drug development is possible without
animal experimentation, and there is still a strong belief that nothing will change. It is
expected that, for the foreseeable future, animal experiments will continue to be performed
to support the development of new drugs using rodent (mice and rats) and non-rodent (dogs
and non-human primates [NHPs]) animal species.
NHPs have been widely used as non-rodent species in nonclinical toxicology studies [3,4,5]. One reason for using NHPs instead of other large
animals such as dogs is that NHPs are genetically similar to humans. Furthermore, when
evaluating certain classes of drug candidates, such as biologics, NHPs are considered the
only pharmacologically appropriate species. However, in February 2022, the US Food and Drug
Administration (FDA) issued guidance restricting the use of NHPs in toxicology studies
[6]. The primary reason for this guidance was to
reserve NHPs for testing investigational COVID-19 treatments and vaccine candidates as the
supply of NHPs was affected by the COVID-19 pandemic. Reducing the number of NHPs used is of
great concern worldwide because it affects the feasibility of non-clinical pharmacological
and toxicological studies, and has developed into a discussion involving various consortiums
and regulatory authorities [7, 8]. This guidance was withdrawn in 2023 with the expiration of the public
health emergency [9]. However, issues remain regarding
the stable supply of NHPs and the impact of a shortage of NHPs on new drug development has
not been completely resolved.
Efforts to reduce the number of animals, not only NHPs, but also other species, such as
rodents, are required in consideration of the 3Rs mentioned above. Although this is not a
complete solution, the concept of virtual control groups (VCGs) was introduced by
Steger-Hartmann et al. in 2020 [10]
to partially contribute to animal reductions. According to their study, VCGs were
constructed from control-group animal data from previously conducted studies that have been
accumulated in a repository. Examples of conditions that are considered to be used for
selection criteria in selecting animals for VCGs by their definition may include, but are
not limited to, animal species/strain, sex, breeder, and age, although this depends on the
evaluation endpoints. By applying a VCG created from historical control data, approximately
30% of the total number of animals could be reduced in repeated-dose toxicology studies
consisting of a control group and three dose groups. Therefore, topics on VCGs, including,
but not limited to, how to construct one, as well as potential issues, are now being
discussed at conferences [11, 12] and international consortia [13,14,15]. The VCG concept was also discussed as a non-clinical topic in PHUSE CSS 2023
[16]. In addition, when the Japan Pharmaceutical
Manufacturers Association (JPMA) presented Taskforce Team activities at the 2022 Fall
Clinical Data Interchange Standards Consortium (CDISC) face-to-face meeting [17], questions from participants focused on the topic of
VCGs, which demonstrated a high level of global interest.
The ultimate goal of pharmaceutical companies is the regulatory acceptance of toxicology
studies applying VCGs while achieving animal population reductions with scientific validity.
However, major issues need to be resolved before VCGs are implemented. For example, can the
effect of the compound under development be appropriately evaluated scientifically, even if
the concurrent control group in the study is replaced with a VCG? Does the application of
VCG pose any problems from a statistical perspective? Will regulatory agencies accept
studies that include no real control animals? We believe that discussing each of these
issues carefully is important. Therefore, the JPMA Taskforce Team, which deals with issues
on safety evaluation in the Non-Clinical Evaluation Expert Committee, Drug Evaluation
Committee, and JPMA, identified various issues that may arise when implementing VCGs and
discussed these issues in detail in this paper.
In this study, VCG refers to a control group virtually created based on study data
collected and accumulated in the past. The contents of the VCG are similar to those of the
historical control data in a test facility; however, the VCG is a subset of the historical
control data. To generate a VCG from historical control data, the required number of animals
was randomly selected from animals that matched conditions such as species, strain, sex,
age, vehicle, and route of administration. Replacing the control group with existing data
has already been partially implemented in the field of clinical trials and is being applied
to trials in which it is difficult to establish a control group owing to recruitment issues
such as rare diseases or ethical considerations [18,
19].
Materials and Methods
Concerns regarding the introduction of VCGs into non-clinical toxicology
studies
The JPMA Taskforce Team on safety evaluation systems (including VCG-related topics)
consists of 16 members with expertise in general toxicology, pathology, reproductive and
developmental toxicology, genotoxicity, immunotoxicology, statistics, and regulatory
agencies. The Taskforce Team discussed concerns about introducing VCGs to non-clinical
toxicology studies from different perspectives at FY2022 to 2023 team meetings. The
remainder of this paper is organized as follows.
1) Regulatory requirements: description of the creation of control groups and dose levels
in guidelines (confirmation of currently valid guidelines).
2) Common issues when introducing VCGs: filtering conditions (conditions that should or
may be matched with treated animals).
3) Issues related to histopathological examinations when introducing VCGs (lot-to-lot
differences in normal tissue images).
4) Statistical analysis when introducing VCGs (differences in the importance of
statistical analysis depending on study type).
5) Facility monitor (sentinel) animals (necessity of monitor animals).
Guidelines examined
The guidelines in Table 1 were examined for
the creation of the control groups.
Table 1. Guidelines examined
| Organization |
Guideline |
Reference |
| ICH |
S4A guideline “Duration of chronic toxicity testing in
animals (rodent and non rodent toxicity testing)” |
[20] |
| OECD |
TG 407 “Repeated dose 28-day oral toxicity study in
rodents” |
[21] |
| TG 408 “Repeated dose 90-day oral toxicity study in
rodents” |
[22] |
| TG 409 “Repeated dose 90-day oral toxicity study in
non-rodents” |
[23] |
| TG 452 “Chronic toxicity studies” |
[24] |
| EMA |
Guideline on repeated dose toxicity |
[25] |
| MHLW |
The partial revision of the guidelines for repeated
dose toxicity studies (Notification No. 655) |
[26] |
EMA: the European Medicines Agency; ICH: the International Council for Harmonisation
of Technical Requirements for Pharmaceuticals for Human Use; MHLW: the Japanese
Ministry of Health, Labour and Welfare; OECD: the Organisation for Economic
Co-operation and Development.
To investigate the distribution of test values in the control group in toxicology
studies, a series of histograms of the distribution of test values in the control groups
of toxicology studies were generated using R version 4.1.0 and R Studio version 1.4.1717.
The results of 10 repeated trials, in which 10 samples were randomly selected from a
normally distributed population, are shown.
Results
Regulatory requirements: description of the creation of control groups and dose
levels in guidelines
The ICH S4A guideline “Duration of chronic toxicity testing in animals (rodent and non
rodent toxicity testing)” [20] specifies the
duration of treatment for chronic toxicology studies in rodents and non-rodents (6 and 9
mo, respectively), along with a discussion of the duration of treatment in non-rodents (6
vs. 12 mo). However, details regarding the design of the toxicology studies are not
provided in the ICH guidelines. In contrast, the OECD repeated-dose toxicology study
guidelines (repeated dose 28-day oral toxicity study in rodents: TG 407 [21]; repeated dose 90-day oral toxicity study in
rodents: TG 408 [22]; repeated dose 90-day oral
toxicity study in non-rodents: TG 409 [23]; chronic
toxicity studies: TG 452 [24]) state that a control
group should be used and the vehicle used to administer the test substance should be
administered to control groups. The EMA guideline “guideline on repeated dose toxicity”
[25] also states that “the treatment should
include appropriate control group (s)”. A partial revision of the guidelines for repeated
dose toxicity studies (Notification No. 655), issued by the MHLW (ex. the Ministry of
Health and Welfare [MHW]) in 1999 [26], similarly
states that “a control group should be set for which the test substance is not
administered (vehicle administration)”. Thus, the current regulatory guidelines on
repeated-dose toxicology studies (OECD, EMA, and MHLW), other than the ICH, describe the
use of a control group to which a vehicle is administered.
Although this is not directly related to introducing VCGs to non-clinical toxicology
studies, there have been some discussions in the Taskforce Team regarding dose levels from
the perspective of reducing the number of animals. TG 408 of the OECD states “if a test at
one dose level equivalent to at least 1,000 mg/kg body weight/day produces no observed
adverse effects and if toxicity would not be expected based upon data from
structurally-related compounds, then a full study using three dose levels may not be
considered necessary”. TG 407 and 409 have similar statements. The dose levels section of
the EMA guideline states “Dose levels may need to be adjusted, in unexpected toxic
responses or lack of responses occurs during the study”. This means that there are
possibilities of both increasing or decreasing the three dose levels, suggesting that
flexibility should be considered depending on the situation. The Japanese MHLW guidelines
do not refer to dosing flexibility.
In some cases, three doses may not be necessary. For example, highly selective biologics
can be administered up to the maximum feasible dose to normal animals without any signs of
toxicity. The EMA and OECD guidelines permit flexibility to adjust such cases, but
conversely, a fourth dose-group may be set as necessary [21,22,23, 25]. The ability to flexibly increase
or decrease the dose levels with scientific appropriateness would lead to a reduction in
the total number of animals. Thus, there is content in the guideline documents that offers
flexibility as long as the number of dose groups goes, and this sort of flexibility may,
with proper justification and health authority agreement, be extended to involve the
utilization of VCGs.
Common issues when introducing VCGs: filtering conditions
When trying to set a VCG in a toxicology study, if the conditions for animal selection
are too strict, there may be insufficient data to use the VCG. Conversely, if the
selection criteria are overly general, inappropriate data may be included. The Taskforce
Team listed the possible filter conditions and used an asterisk (*) to indicate those that
should be matched (i.e., critical ones) in Table
2. The basis for setting these conditions were set to minimize the variation in
the normal values in the control group. Our idea is similar to that reported by Aulbach
et al. [27] regarding factors
that influence clinical pathology data when attempting to distinguish between test
article- and non-test article-related effects in non-clinical safety studies; the listed
factors overlapped with each other. According to this report, red blood cell parameters
(erythrocyte count, hemoglobin concentration, and hematocrit percentage) and white blood
cell composition (e.g., lymphocytes and neutrophils) change with aging in rats and dogs.
In terms of the dosing route, there is a possibility that parameters such as AST and ALT
may be slightly elevated owing to tissue damage caused by intravenous administration.
Thus, these values cannot be combined across studies as control group values unless
certain conditions are unified. Furthermore, some parameters may vary depending on the
animal supplier and/or breeding conditions [28],
including the frequency of pathological changes in corneal mineralization in rats [29]. However, cases of such variations have rarely been
reported.
Table 2. Virtual control groups (VCG) filter conditions
| • Species and strain* |
| • Sex* |
| • Age (with a certain range)* |
| • Test facility |
| • Breeder |
| • Origin of the animal |
| • Breeding conditions (including food and drinking water) |
| • Dosing route |
| • Vehicle |
| • Clinical laboratory equipment |
| • Possible genetic variation (e.g., conducted within 5 years for
rodents) |
*Conditions that the Taskforce Team think should be matched (i.e., critical
ones).
Technologies, such as the digitization of pathological slides and machine learning of
normal/abnormal tissue images for artificial intelligence (AI) pathological diagnosis, are
advancing globally [30]. The JPMA also launched
another Taskforce Team on AI pathology (hereafter referred to as the AI pathology
Taskforce Team) in FY2023. This AI pathology Taskforce Team initiated activities with the
aim of building a specific system in cooperation with related organizations, rather than
simply collecting information. Given these circumstances, it is highly likely that
abnormal histopathological findings caused by compound administration can be diagnosed
using a database of background control data. However, compared to the clinical pathology
data of the control groups, histopathological data are image data and could be large in
size, and a one-slide image may contain both normal and different types of abnormal
regions. Furthermore, dyeability may differ depending on the facility, making it difficult
to handle the data.
Upon discussion within the Taskforce Team, it was noted that pathologists maintained that
reading the slides of control animals within a particular study is valuable. This is
especially true for small animals, as there may be lot-to-lot differences in normal tissue
images in the control group, such as frequency of spontaneous corneal and lens opacity in
rats [31, 32]. At this time, no conclusion can be drawn regarding this point, although
pathologists are conducting histopathological analysis. However, advances in this field
may occur with the creation of an AI pathological diagnosis system that considers
lot-to-lot differences. If the issues mentioned above are resolved, the situation in which
histopathological examination becomes the rate-limiting factor for introducing VCGs to
non-clinical toxicology studies will be avoided.
Statistical analysis when introducing VCGs
It is assumed that multiple 4-week repeated-dose toxicology studies (at least three
animals per sex per group for large animals and 10 animals per sex per group for small
animals), including concurrent controls, even if repeated under exactly the same
conditions, would likely result in different statistical outcomes each time. It is
possible that the replacement of control groups with VCGs may also result in different
statistical outcomes because the groups to be compared are different. Additionally, as the
number of animals in the virtual control group increased, the number of endpoints that
were statistically significant also tended to increase. Therefore, the statistical
significance of these findings must be considered.
This is related to the interpretation of statistical analysis results in the evaluation
of short-term repeated-dose toxicology study results. Although appropriate statistical
methods are required as per the OECD and EMA guidelines [21,22,23,24,25], we believe that it is useful to consider all study data in addition to
statistical significance when evaluating a study. A statistically significant difference
suggests the effects of the test compound, and comprehensive conclusions should be drawn
based on the results of other endpoints, including the histopathological examination. This
is different from clinical trials or carcinogenicity among non-clinical studies, which
emphasize statistical results. Conversely, even if there were no statistically significant
differences, the findings were judged as toxicologically significant. Therefore, the
objective of this study can be achieved if a VCG that can detect potential toxic findings
is used with a reduced number of animals.
In a typical 4-week repeated-dose toxicology study in rodents, the number of animals per
group is 10 per sex, as is the number of animals in the control group. As discussed in
“Investigation on the distribution of test values in the control group in toxicology
studies” described later, even clinical pathology data (e.g., glucose in this case) are
normally distributed in the population, but in 10 samples, although the mean values were
similar, the distributions differed depending on the trials. In addition, the standard
deviations varied considerably depending on the study. If this statistically natural
variation also occurs in toxicology studies, the statistically significant difference
shown by the 10 control group values cannot be considered definitive. As mentioned above,
toxicology assessments should be performed comprehensively with other parameters while
referring to statistical differences as a component of the analysis.
Facility monitor (sentinel) animals
One of the roles of concurrent control groups in repeated-dose toxicology studies is to
determine the influence of factors other than the effects of the test compound (e.g.,
breeding environment). The introduction of the VCG may affect the ability to identify
these external factors. Particularly in long-term studies, it is possible to use “facility
monitor animals” for this purpose in addition to study animals. The OECD TG 452 [24] states: “In chronic toxicity studies, additional
groups of sentinel animals may also be included for the monitoring of disease status, if
necessary, during the study”. The use of facility monitor animal is not always essential.
However, the use of such animals in the animal room should be considered to eliminate
suspicion of infectious diseases not caused by the administration of the test compound in
long-term rearing, even if VCG is introduced in the future.
Investigation on the distribution of test values in the control group in toxicology
studies
Some human clinical test results were normally distributed. According to Okubo [33], normally distributed parameters include serum
sodium, calcium, chloride, inorganic phosphorus, total protein, albumin, cholinesterase,
uric acid, and glucose.
Since we know from collective experience that serum sodium in rats has a very narrow
distribution of values, we selected glucose as an example, which has a relatively wider
distribution of values. A simplistic model was created with the assumption that blood
glucose levels in rats were normally distributed, as in humans. The R programming language
was used to generate 100,000 random numbers with a mean of 250 and a standard deviation of
30 (Fig. 1). Ten samples were then selected from
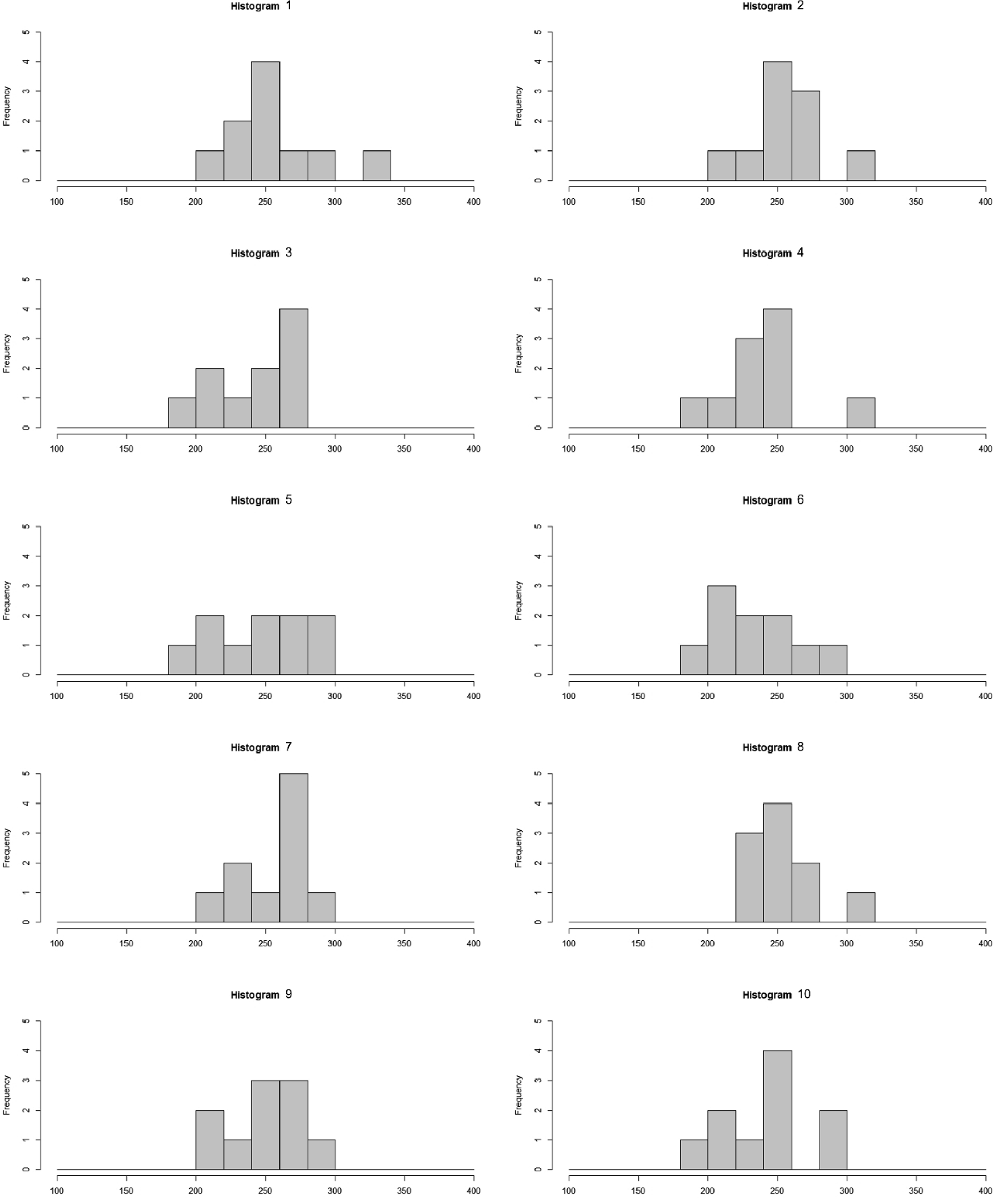
these random numbers, which were repeated 10 times, and the distribution was examined
(Fig. 2). Sampling of 10 was intended to mimic
the number of animals per sex per group in short-term (e.g., 4-week) repeated dose
toxicology studies in rodents, as mentioned above. Means and standard deviations for each
trial were calculated (Table 3).
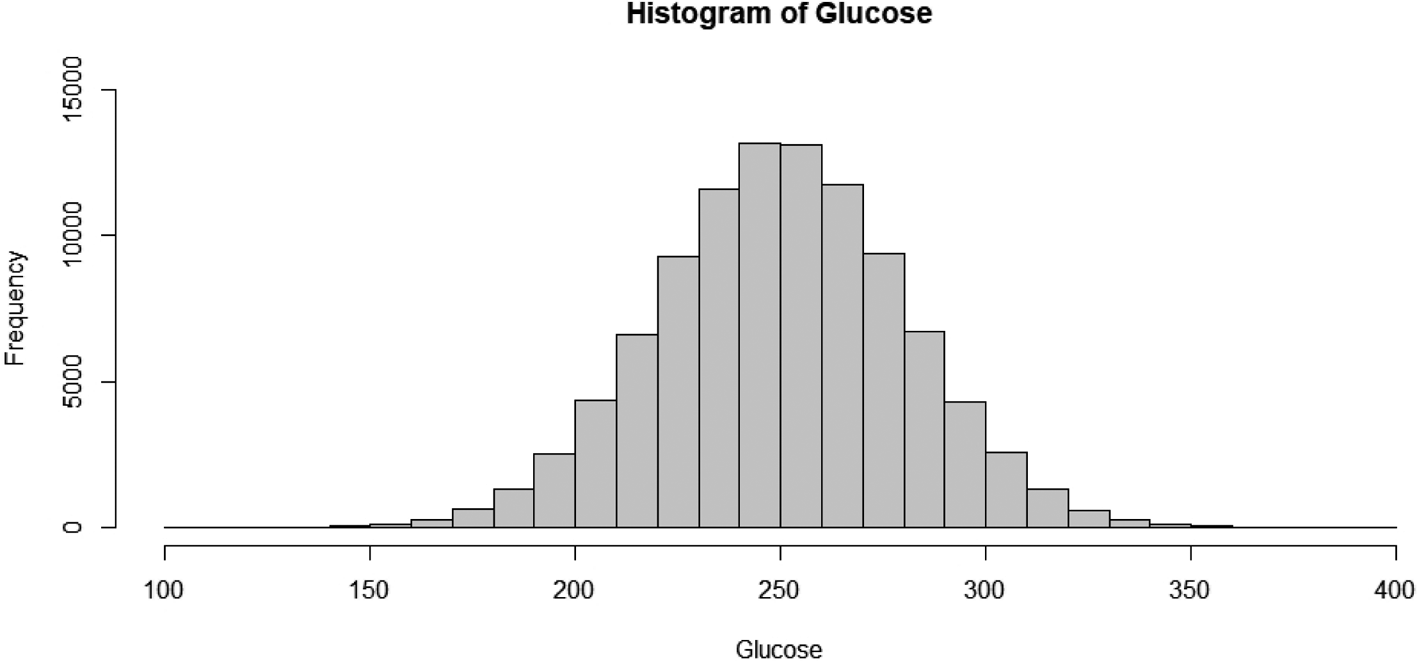
Table 3. Mean and standard deviation (SD) values for 10 trials
| Trial no. |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
| Mean |
258.6 |
254.4 |
243.3 |
244.5 |
248.6 |
231.9 |
256.6 |
253.9 |
251.7 |
243.1 |
| SD |
31.5 |
25.5 |
27.6 |
27.9 |
32.8 |
31.5 |
27.1 |
25.1 |
25.8 |
36.7 |
The R code used was as follows:
When n=10 sampling was repeated 10 times, the average value was generally similar to that
of the population, but the standard deviation was different each time, and the
distribution was biased such that it could not be said to be a normal distribution. There
are simplified examination results using R programming, but we can obtain results similar
to those of the simulation if we repeat this process of randomly extracting 10 samples
from the accumulated glucose data (data not shown). The similarity between the created
glucose model and the actual glucose data supports the use of VCGs. Despite the use of
VCG, it should be noted that appropriate toxicology studies should take the historical
control data of the facility, and the results of histopathological examinations should be
considered.
Discussion
Kawaguchi et al. [12]
retrospectively introduced VCG into a 4-week repeated-dose toxicology study in rats that had
already been evaluated and examined. Candidate animals for VCG were selected from previous
studies with matching conditions, such as study animals (e.g., species, strain, sex, and
age), experimental conditions, and measurement equipment. Body weight was selected as the
selection criterion for retrospective VCG analysis. Body weights at the time of grouping
should be similar so that changes can be evaluated. That is, the VCG is set as within (a)
the mean ± SD or (b) the mean ± 2SD relative to the original control group (i.e., concurrent
control group). The number of animals in the VCG group was 10, which was the same as that in
the compound treatment groups. A statistical significance test was performed between the
original control group or VCG group and the treatment groups to detect the effects of the
compound. Results of this statistical analysis in the evaluation of body weight changes
indicated that when (a) mean ± SD was selected for the VCG, a statistically significant
difference was shown, similar to when using concurrent controls as expected. Similar results
were also obtained when (a) mean ± SD or (b) mean ± 2SD based on the body weight at grouping
was selected for the VCG in evaluating hematology and blood chemistry tests. In other words,
while some of the results when using the concurrent control were reproduced, some items
showed new statistically significant differences when compared with the VCG. Conversely,
some items lost statistical significance when compared with the VCG. However, these results
should not completely preclude the use of VCGs in toxicology studies, as there could be
variations between the actual control groups in the two different studies conducted in the
same manner. The use of VCGs should continue to be explored, especially in certain types of
studies, such as dose-range-finding or short-term repeated dose toxicology studies. However,
some endpoints such as histopathological evaluations may have specific concerns.
Another important point that may be raised when discussing the introduction of VCGs is
revisiting current evaluation methods for toxicology studies (especially short-term
repeated-dose toxicology studies). Many toxicologists evaluate toxicology study results
regarding their toxicological significance by referring to statistically significant
differences in body weight changes and clinical pathology results after confirming related
histopathological changes, and then comprehensively draw final conclusions. There is a
concern that completely consistent statistical results may not be obtained by replacing a
concurrent control group with VCG. However, this does not imply that the statistical power
will change. This is because, as shown in the simulation of glucose values, if there are 10
animals of each sex per group, the distribution of values in the concurrent control group
may be biased by chance (the mean value of Trial #6 is low). By performing a comprehensive
evaluation, including histopathological changes, the impact of the introduction of VCGs
should be minimized in terms of toxicological significance.
To address this point, Wright et al. [34] conducted a larger investigation by retrospectively analyzing the impact of
replacing concurrent control groups with VCGs on the treatment relatedness of
histopathological findings in the eTOX database. The results suggested that VCGs could be
used most successfully when only incorporating historical control data that had strong
similarity to concurrent control groups, that is, the same species, strain, sex,
administration route, and vehicle. These results support our discussion (Table 2) and are helpful in the selection of the VCG filter
conditions.
Specifically related to historical control data, Kluxen et al. [35] performed a very detailed review of statistical tests
for the use of historical control data in the evaluation of toxicology study results.
According to their study, statistical approaches can be used to set historical control
limits or relevance thresholds based on the historical control data. Statistical approaches
can be used to compare concurrent responses to derived relevance thresholds. If a value
exceeds a relevance threshold, the response may be abnormal or considered biologically
irrelevant. However, they stated that this should be considered as one piece of evidence
contributing to, but not replacing, toxicological assessments.
General toxicology studies, particularly short-term repeated-dose toxicology studies, are
discussed in this manuscript. However, the use of VCGs may also be applicable to other types
of studies. It is necessary to consider the usefulness of VCGs in terms of evaluating
endpoints, such as electrocardiogram (ECG) and respiratory data in safety pharmacology
studies, which can be modeled in a standard format by implementing the latest CDISC Standard
for Exchange of Nonclinical Data (SEND) implementation guide (IG) Version 3.1.1 [36]. The Taskforce Team believed that these possibilities
require further discussion.
In the near future, the accumulation of such evidence will support the appropriate use of
VCGs. However, if VCGs are to be used, acceptance by regulatory authorities is of utmost
importance. Although this is not directly related to the introduction of VCGs in
non-clinical toxicology studies, there have been some discussions regarding dose levels from
the perspective of reducing the number of animals. As mentioned in the Results section
“Regulatory requirements: description of the creation of control groups and dose levels in
guidelines”, the currently effective regulatory guidelines on repeated dose toxicology
studies (OECD, EMA, MHLW), other than the ICH, describe the use of a control group to which
the vehicle is administered. In addition, as for the dose level, 3 doses are basically set,
which can be adjusted flexibly in the EMA and OECD guidelines. As discussions on the
introduction of VCGs progress and from the perspective of animal welfare, it will be
important to update the guidelines accordingly. The active participation of regulatory
authorities in VCG discussions is extremely valuable and strongly encouraged.
Assuming that VCGs are scientifically justified and regulatory authorities accept
toxicology studies with study designs that include VCGs, sponsors of non-clinical studies
may need to consider their interactions with CROs regarding whether to include a VCG in the
study design. Consequently, CROs may be required to enrich their facilities’ historical
control data under various conditions to create an IT environment that facilitates the use
of VCGs. To achieve this, it may be useful to use data accumulated in the SEND format, which
is a non-clinical CDISC standard, and extract the appropriate data. However, this approach
needs to be discussed separately and extensively, as the SEND data may contain information
on other sponsors (i.e., information on the original study).
Conclusions
In recent years, the concept of VCGs has spread rapidly, and is now being discussed
globally. In this study, we identified issues that may arise when implementing VCG and
explored them. The issues discussed in this paper, namely regulatory requirements, common
issues when introducing a VCG, issues related to histopathological examination when
introducing a VCG, statistical analysis when introducing a VCG, and facility monitor
(sentinel) animals, are examples that will arise with the introduction of VCGs, and
unexpected issues are anticipated. Additionally, the introduction of the VCG concept may
impact the current evaluation methods for toxicology studies. Although the introduction of
VCGs stems from a reduction in the number of animals from the viewpoint of the 3Rs and the
problem of NHP supply, with the increasing utilization of data in standard formats, the VCG
concept is even more likely to gain traction. Even if the use of VCGs is justified from
animal welfare and scientific perspectives, it is critical that the industry works with
health authorities to ensure that data from studies utilizing VCGs are accepted. The JPMA
will continue to hold the necessary discussions with key stakeholders to accelerate
efficient and effective new drug development pertaining to the use of VCGs. Furthermore, the
JPMA would also be involved in discussions on the international harmonization of study
designs implementing VCGs (e.g., how to choose appropriate animals for VCGs and the animal
number of VCGs).
Conflict of Interest
The authors (Gen Sato, Mikio Nakajima, Kuniyoshi Sakai, Yuko Togashi, Masakatsu Yamamoto,
Yuki Inoue, Takeshi Oshima, Tetsuyoshi Soh, Mayumi Watanabe, Izumi Matsumoto, Toshinobu
Yamamoto, Takashi Tanaharu, Akio Kawakami, Keiko Motoyama, Kiyohiro Hashimoto, and Mutsumi
Suzuki) are employees of companies (Eisai Co., Ltd., Asahi Kasei Pharma Corporation, ASKA
Pharmaceutical Co., Ltd., EA Pharma Co., Ltd., MSD K.K., Otsuka Pharmaceutical Co., Ltd.,
Kyowa Kirin Co., Ltd., Shionogi & Co., Ltd., Daiichi Sankyo Co., Ltd., Sumitomo Pharma
Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Bristol-Myers Squibb K.K., Merck Biopharma
Co., Ltd., Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co. Limited, and Kyowa Kirin
Co., Ltd.) who are funding the research.
Acknowledgements
We are grateful to the former Taskforce Team member Kyohei Nishimura (Shionogi & Co.,
Ltd.), who was replaced during the research period, for his valuable contributions to our
research work. We would like to thank Shun Kawaguchi (Mediford Corporation) for his multiple
discussions of VCGs based on their implementation using a retrospective approach. We would
also like to thank all the reviewers of the Taskforce Team member companies for their
thorough reviews and useful suggestions in the preparation of this manuscript. Furthermore,
we wish to acknowledge Thomas Visalli (Eisai Inc.) for providing valuable feedback during
the preparation of this manuscript and for improving its language quality.
References
- 1. Gorzalczany, S.
B. and Rodriguez Basso,
A. G.
2021. Strategies to apply 3Rs in preclinical
testing. Pharmacol. Res. Perspect.
9: e00863.
- 2. Wadman,
M.
2023. FDA no longer needs to require animal tests before human drug trials.
Science Insider.
- 3. EU: European Union
2009. Non-human primates in research and safety testing. https://ec.europa.eu/health/scientific_committees/opinions_layman/en/non-human-primates/index.htm
[accessed August 23, 2023].
- 4. Prior,
H., Haworth,
R., Labram,
B.,
Roberts, R.,
Wolfreys,
A. and
Sewell,
F.
2020. Justification for species selection for pharmaceutical
toxicity studies. Toxicol. Res.
(Camb.)
9: 758–770.
- 5. Monticello, T.
M., Jones,
T. W.,
Dambach, D.
M., Potter,
D. M.,
Bolt, M. W.,
Liu, M.,
Keller, D.
A., Hart, T.
K. and Kadambi,
V. J.
2017. Current nonclinical testing paradigm enables safe entry
to First-In-Human clinical trials: the IQ consortium nonclinical to clinical
translational database. Toxicol. Appl.
Pharmacol.
334: 100–109.
- 6. FDA: US Food and Drug Administration.
2022. Nonclinical Considerations for Mitigating Nonhuman Primate Supply
Constraints Arising from the COVID-19 Pandemic Guidance for Industry. Federal Register
87(37), 10373–10375. https://www.govinfo.gov/content/pkg/FR-2022-02-24/pdf/2022-03915.pdf
[accessed August 23, 2023].
- 7. NC3Rs. 2022. Webinar: Minimising
non-human primate use in drug development. https://www.nc3rs.org.uk/events/minimising-NHP-use [accessed August 23,
2023].
- 8. Ackley,
D., Birkebak,
J., Blumel,
J.,
Bourcier,
T., de Zafra,
C.,
Goodwin, A.,
Halpern,
W., Herzyk,
D.,
Kronenberg,
S., Mauthe,
R.,
Shenton, J.,
Shuey, D.
and Wange, R.
L.
2023. FDA and industry collaboration: identifying
opportunities to further reduce reliance on nonhuman primates for nonclinical safety
evaluations. Regul. Toxicol.
Pharmacol.
138: 105327.
- 9. Brown, P.
C. and Wange,
R. L.
2023. Considerations regarding the use of nonhuman primates in
assessing safety endpoints for pharmaceuticals. Regul.
Toxicol. Pharmacol.
143: 105449.
- 10. Steger-Hartmann,
T.,
Kreuchwig,
A., Vaas,
L.,
Wichard, J.,
Bringezu,
F., Amberg,
A., Muster,
W., Pognan,
F. and
Barber,
C.
2020. Introducing the concept of virtual control groups into
preclinical toxicology testing. Altern. Anim.
Exp.
37: 343–349.
- 11. Andaya, R.
V., Pourmohamad,
T.,
Sullivan,
R., Hayes,
M. and
Anger,
L.
2023. Virtual Control Groups in Preclinical Safety: Decreasing Animal Use
while Maintaining In Vivo Study Interpretability. The 62nd Annual Meeting of the Society
of Toxicology.
- 12. Kawaguchi,
S.,
Takahashi,
K. and
Yamamoto,
D.
2023. Introduction of Virtual Control Group in Nonclinical safety studies.
The 50th Annual Meeting of the Japanese Society of Toxicology.
- 13. eTRANSAFE. 2022. eTRANSAFE announces
the launch of the “eTRANSAFE ViCoG Initiative” https://etransafe.eu/etransafe-announces-the-launch-of-the-etransafe-vicog-initiative/
[accessed August 23, 2023].
- 14. Sanz,
F., Pognan,
F.,
Steger-Hartmann,
T., Díaz,
C.,
Asakura, S.,
Amberg, A.,
Bécourt-Lhote,
N., Blomberg,
N., Bosc,
N., Briggs,
K.,
Bringezu,
F.,
Brulle-Wohlhueter,
C., Brunak,
S.,
Bueters, R.,
Callegaro,
G.,
Capella-Gutierrez,
S., Centeno,
E., Corvi,
J., Cronin,
M. T. D.,
Drew, P.,
Duchateau-Nguyen,
G., Ecker,
G. F.,
Escher, S.,
Felix, E.,
Ferreiro,
M., Frericks,
M.,
Furlong, L.
I., Geiger,
R., George,
C.,
Grandits,
M.,
Ivanov-Draganov,
D.,
Kilgour-Christie,
J.,
Kiziloren,
T., Kors,
J. A.,
Koyama, N.,
Kreuchwig,
A., Leach,
A. R.,
Mayer, M.
A., Monecke,
P., Muster,
W.,
Nakazawa, C.
M., Nicholson,
G., Parry,
R., Pastor,
M., Piñero,
J.,
Oberhauser,
N.,
Ramírez-Anguita, J.
M., Rodrigo,
A., Smajic,
A.,
Schaefer,
M.,
Schieferdecker,
S., Soininen,
I.,
Terricabras,
E.,
Trairatphisan,
P., Turner,
S. C.,
Valencia,
A., van de
Water, B.,
van der Lei, J.
L., van Mulligen,
E. M.,
Vock, E. and
Wilkinson,
D.
2023. eTRANSAFE: data science to empower translational safety
assessment. Nat. Rev. Drug Discov.
22: 605–606Comment.
- 15. TransCelerate
2023. The use of virtual controls in preclinical research: promise and
predicament. https://www.transceleratebiopharmainc.com/events/the-use-of-virtual-controls-in-preclinical-research-promise-and-predicament/
[accessed August 23, 2023].
- 16. PHUSE CSS
2023, Nonclinical topics. 2023. https://www.phuse-events.org/attend/frontend/reg/tOtherPage.csp?pageID=15403&ef_sel_menu=1694&eventID=26
[accessed August 23, 2023].
- 17. Sato,
G.
2022. Updates from the JPMA SEND team. 2022 Fall CDISC Face-to-Face
Meeting.
- 18. Nakatsuka,
Y.
2019. Utilization of clinical trial control groups in clinical development.
Office of Pharmaceutical Industry Research News 58 https://www.jpma.or.jp/opir/news/058/pdf/pdf-13–01.pdf [accessed August 23,
2023] (in Japanese).
- 19. Gökbuget,
N., Kelsh,
M., Chia,
V., Advani,
A., Bassan,
R.,
Dombret, H.,
Doubek, M.,
Fielding, A.
K., Giebel,
S., Haddad,
V.,
Hoelzer, D.,
Holland,
C., Ifrah,
N., Katz,
A., Maniar,
T.,
Martinelli,
G., Morgades,
M.,
O’Brien, S.,
Ribera, J.
M., Rowe, J.
M., Stein,
A., Topp,
M.,
Wadleigh,
M. and
Kantarjian,
H.
2016. Blinatumomab vs historical standard therapy of adult
relapsed/refractory acute lymphoblastic leukemia. Blood
Cancer J.
6: e473.
- 20. ICH: The International Council for
Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
S4A: Duration of Chronic Toxicity Testing in Animals (Rodent and Non Rodent Toxicity
Testing) https://database.ich.org/sites/default/files/S4_Guideline.pdf [accessed
August 23, 2023].
- 21. OECD: Organisation for Economic
Co-operation and Development
2008. Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents. OECD
Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. https://www.oecd-ilibrary.org/environment/test-no-407-repeated-dose-28-day-oral-toxicity-study-in-rodents_9789264070684-en
[accessed August 23, 2023].
- 22. OECD
2018. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents. OECD
Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. https://www.oecd-ilibrary.org/environment/test-no-408-repeated-dose-90-day-oral-toxicity-study-in-rodents_9789264070707-en
[accessed August 23, 2023].
- 23. OECD
1998. Test No. 409: Repeated Dose 90-Day Oral Toxicity Study in Non-Rodents.
OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. https://www.oecd-ilibrary.org/environment/test-no-409-repeated-dose-90-day-oral-toxicity-study-in-non-rodents_9789264070721-en
[accessed August 23, 2023].
- 24. OECD
2018. Test No. 452: Chronic Toxicity Studies. OECD Guidelines for the Testing
of Chemicals, Section 4. OECD Publishing, Paris. https://www.oecd-ilibrary.org/environment/test-no-452-chronic-toxicity-studies_9789264071209-en
[accessed August 23, 2023].
- 25. EMA: European Medicines
Agency
2010. Guideline on repeated dose toxicity. CPMP/SWP/1042/99 Rev 1 Corr.
https://www.ema.europa.eu/en/repeated-dose-toxicity-scientific-guideline
[accessed August 23, 2023].
- 26. MHLW: Japanese Ministry of Health,
Labour and Welfare. 1999. Partial Revision of the Guidelines
for Repeated Dose Toxicity Studies (Notification No. 655) https://www.pmda.go.jp/files/000156632.pdf [accessed August 23, 2023]
(in Japanese).
- 27. Aulbach,
A.,
Provencher,
A. and
Tripathi,
N.
2017. Influence of study design variables on clinical
pathology data. Toxicol. Pathol.
45: 288–295.
- 28. Shimomura,
K.
2018. 5.5 Developmental and reproductive toxicology in Toxicology, 3rd ed.
Asakura Publishing Co., Ltd., Tokyo, Japan. pp.156–165 (in
Japanese).
- 29. Hashimoto,
S., Doi,
T., Wako,
Y., Sato,
J., Wada,
S. and
Tsuchitani,
M.
2013. Corneal mineralization in wistar hannover
rats. J. Toxicol. Pathol.
26: 275–281.
- 30. BIGPICTURE
2023. https://bigpicture.eu/ [accessed August 17, 2023].
- 31. Onodera,
H., Sasaki,
S., Otake,
S.,
Tomohiro,
M., Shibuya,
K. and
Nomura,
M.
2013. Scientific viewpoints in ocular toxicity assessment:
departure from conventional practice. Anim Eye
Res.
32: 3–13(in
Japanese).
- 32. Onodera,
H., Sasaki,
S., Otake,
S.,
Tomohiro,
M., Shibuya,
K. and
Nomura,
M.
2015. General considerations in ocular toxicity risk
assessment from the toxicologists’ viewpoints. J.
Toxicol. Sci.
40: 295–307.
- 33. Okubo,
A.
1993. 2. How to read normal and abnormal
values. J Jpn Soc Int Med
82: 485–489(in
Japanese).
- 34. Wright, P. S.
R., Smith,
G. F.,
Briggs, K.
A., Thomas,
R.,
Maglennon,
G.,
Mikulskis,
P., Chapman,
M., Greene,
N.,
Phillips, B.
U. and Bender,
A.
2023. Retrospective analysis of the potential use of virtual
control groups in preclinical toxicity assessment using the eTOX
database. Regul. Toxicol. Pharmacol.
138: 105309.
- 35. Kluxen, F.
M., Weber,
K., Strupp,
C., Jensen,
S. M.,
Hothorn, L.
A., Garcin,
J. C. and
Hofmann,
T.
2021. Using historical control data in bioassays for
regulatory toxicology. Regul. Toxicol.
Pharmacol.
125: 105024.
- 36. CDISC
2021. Standard for Exchange of Nonclinical Data Implementation Guide
(SENDIG): Nonclinical Studies. Version 3.1.1 (Final). https://www.cdisc.org/standards/foundational/send/sendig-v3-1-1 [accessed
November 16, 2023].