Article ID: 2024-001
Article ID: 2024-001
Medication history is a series of data for each patient recorded by pharmacists in daily clinical practice in Japanese pharmacies. This real-world data potentially contains useful information on various risks induced by drugs; however, this information has rarely been used. Therefore, we aimed to verify whether medication histories can be used for drug-induced risk assessment by comparing them with known events as references. To this end, we chose previously reported large-scale trials indicating that anticholinergic drugs for overactive bladder (OAB) are associated with the risk of inducing dementia. We referred to these studies and conducted a retrospective study based on the medication histories of 172,958 patients aged 55 years or older visiting a community pharmacy. Six drugs for OAB (oxybutynin, propiverine, tolterodine, fesoterodine, solifenacin, and imidafenacin) were tested for their risk of inducing dementia, which was determined at the start of using one of the four drugs for dementia (donepezil, galantamine, rivastigmine, and memantine). The association between OAB medications and dementia was assessed using odds ratios (ORs) and 95% confidence intervals (95% CIs). The analysis included 2,634 patients in the case group and 170,324 patients in the control group. A significant difference was observed between the case and control groups (OR, 2.12; 95% CI, 1.66–2.67), indicating that anticholinergic drugs increased the risk of developing dementia. The results were equivalent to those of the referenced large-scale clinical trials, suggesting that medication histories are useful for drug-induced risk assessment.
We conducted a retrospective study based on the medication histories of 172,958 patients 55 years or older visiting a community pharmacy. Six drugs for overactive bladder (OAB) were tested for the risk of inducing dementia. The association between OAB medications and dementia was assessed, and a significant difference was observed between the OAB-treated group and the control group (odds ratio, 2.12; 95% confidence interval, 1.66–2.67). This indicated that anticholinergic drugs had an increased risk of inducing dementia. The results aligned with those of previous large-scale clinical trials, suggesting that medication histories are useful for drug-induced risk assessment.
Recent technological innovations have led to the development of several drugs with novel mechanisms of action, which have different pharmacological actions and safety profiles from those of existing drugs. In addition, with the rapid aging of the Japanese population, adverse events related to polypharmacy among older adults have become a cause for concern, making the strengthening of post-marketing safety measures for pharmaceuticals an important issue [1,2,3].
The Food and Drug Administration Adverse Event Reporting System (FAERS) and the Japanese Adverse Drug Event Report (JADER) databases of the Pharmaceuticals and Medical Devices Agency (PMDA) and the Medical Information Database Network (MID-NET) of PMDA, based on electronic medical records and receipt information, exist as basic information databases for adverse event evaluation used in the post-marketing surveillance of pharmaceutical products. However, the FAERS and JADER are based on spontaneous reports that do not cover the entire patient population and are subject to various biases. MID-NET is rich in information but contains a relatively small amount of data, and the population bias (age, diseases, and medications) is large. In addition, there is little information on the patients’ subjective side effects. Therefore, a database that covers the entire patient population and accumulates subjective and objective patient information is necessary as a complementary drug safety evaluation infrastructure [4, 5]. Therefore, we focused on using medication history as a database.
Medication history is the record of each patient visiting a pharmacy in Japan and includes drug preparation, drug administration instructions, basic information about the patient, their general health and illnesses [6]. More than 60,000 dispensing pharmacies in Japan issue approximately 800 million prescriptions annually, generating a vast medication history [7]. Medication history has generality and completeness, as well as information on outcomes, which are not found in other forms of medical information; for example: (1) it covers the majority of patients with a wide range of illnesses, age groups, and home and older adult care facilities; (2) it includes subjective information about patients obtained through dialogue allowing evaluation of their physical condition and any side effects; (3) it records drug administration status and lifestyle habits from a pharmacological perspective; and (4) it allows continuous information collection from a single patient.
Notwithstanding the above, there has been little proactivity in using medication history for drug safety evaluations. One reason is that an environment for utilizing real-world data sources, such as medication history, has not been developed regarding data quality control, standardization, and other factors [8].
Anticholinergic drugs block muscarinic acetylcholine receptors and are used clinically as antihistamines, antidepressants, and in the treatment of gastrointestinal disorders and overactive bladder (OAB). Based on their mechanism of action, large-scale clinical trials have shown that long-term use of these drugs is associated with an increased risk of cognitive dysfunction and dementia in older individuals, which increases with increasing anticholinergic drug exposure [9,10,11,12].
In this study, we compared data from patients’ medication history with the results of clinical trial reports on the increased risk of developing dementia due to OAB medication to determine whether an increased risk of adverse events could be detected. Based on the medication history data, we observed that anticholinergic drugs increased the risk of developing dementia. These results are equivalent to those of previous large-scale clinical trials.
This retrospective study was conducted using data from the medication history database of patients who visited 15 branches of Sundrug Co., Ltd., a community pharmacy. This database contained information on all drugs prescribed during the study period, including the generic name, date of dispensing, amount dispensed (number of tablets or total liquid volume, tablet specification, or liquid concentration), route of administration, unused medication, changes in the patient’s physical condition, concomitant medications, medical history, visits to other departments, side effects, preferences, and other generic medications. The study was conducted in accordance with the Japanese Personal Information Protection Law and the Ethical Guidelines for Medical and Health Research Involving Human Subjects, with the approval of the Teikyo University Ethical Review Board for Medical and Health Research Involving Human Subjects (approval number: 21-138-3).
ParticipantsThe study period for the medication history database was from January 1, 2013, to March 31, 2022. The participants were patients with dementia aged 55 years or older at the beginning of the observation period. The excluded individuals were 1) patients who had been seen less than twice; 2) patients whose medical history included Huntington’s disease, Parkinson’s disease, Creutzfeldt–Jakob disease, or HIV infection; and 3) patients whose dementia medications were prescribed before the first OAB medication prescription date.
The drugs included in the study were six approved for manufacturing and marketing in Japan for treating OAB (oxybutynin hydrochloride, propiverine hydrochloride, tolterodine tartrate, fesoterodine fumarate, solifenacin succinate, and imidafenacin) which have anticholinergic effects, four drugs for dementia (donepezil hydrochloride, galantamine hydrobromide, rivastigmine, and memantine hydrochloride), and various other branded and generic drugs (96 drugs for OAB and 361 drugs for dementia). The study design was based on a previous report [11], and the anticholinergic drugs included were the same except for darifenacin, trospium (not listed in the National Health Insurance Drug Price List), imidafenacin, and flavoxate. First-line anticholinergic agents (recommended grades A to B) in the domestic guidelines for OAB treatment were selected as target drugs considering their clinical use, and flavoxate, which has a lower recommended grade, was removed from the list. As the only disease covered by dementia drugs was dementia, dementia onset caused by OAB medication was defined as the initiation of a dementia drug prescription after the prescription of an OAB drug in the medication history record. This definition excluded cases where the dementia medication was prescribed before the OAB medication or if the dementia medication was initiated before the medication history study period.
Patients who received an additional prescription for dementia medication after starting treatment with OAB medication were defined as the dementia case group, and those who did not receive an additional prescription were defined as the control group.
Based on medication history, the study participants’ prescription dates, dosages, and days of treatment with medications for OAB and dementia were investigated. Patient background information, such as age and sex, was also investigated.
CalculationThe odds ratio (OR) for developing dementia in the case and control groups was calculated as the primary endpoint. Risk ratios for developing dementia were compared with previously reported large-scale clinical trials to validate the drug safety assessment method based on medication history information. Table 1 shows the primary descriptive characteristics of the four eligible studies, including the country, study design, database, sample size, age, and maximum follow-up period.
| References | Country | Study design | Database | Sample size | Population | Maximum follow-up (years) | Age, median (IQR), years | Male sex (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Case Patients | Controls | Case Patients | Controls | |||||||
| Gray et al. 2015 [10] | USA | Prospective cohort | Integrated health care delivery system in Seattle | 3,434 | Community population aged ≥65 | 10 | 75 (70–80) | 73 (69–78) | 37 | 52 |
| Coupland et al. 2019 [11] | UK | Nested case control | QResearch primary care database | 284,343 | Primary care patients aged ≥55 | 20 | 82 ± 7 (mean ± SD) | 82 ± 7 (mean ± SD) | 37 | 37 |
| Malcher et al. 2022 [12] | France | Nested case control | French National Medical Administrative Database | 86,623 | Primary care patients aged ≥60 | 10 | 82 (72–92) | 82 (72–92) | 33 | 33 |
| OAB casesa | OAB cases | |||||||||
| Dementia | Not dementia | Dementia | Not dementia | |||||||
| Current report | Japan | Retrospective cohort | Medication history database of the Sundrug Co., Ltd. | 172,958 | Patients aged ≥55 | 9 | 80 (70–80) | 70 (60–75) | 46 | 46 |
aAge of medication history devided into age categories spanning 5-year increments. IQR: inter quartile range: SD: standard deviation; OAB: overactive bladder.
Statistical analyses were performed using R software (version 4.2.3; R Foundation for Statistical Computing, Vienna, Austria). The ORs and 95% confidence intervals (CIs) were calculated for the risk of developing dementia in the case group relative to the control group.
The purpose of this study was to evaluate the reliability of medication history as a database and its potential use in drug safety evaluation methods by evaluating an existing event, i.e., the increased risk of developing drug-induced dementia due to anticholinergic drugs.
During the study period, 451,412 patients visited 15 Sundrug Co., Ltd. community pharmacy branches. Of these, 172,958 medication histories of patients aged 55 years or older at the beginning of the observation period were included in the study (Fig. 1). Of the 172,958 patients studied, 2,634 were prescribed an OAB medication (OAB group), and 170,324 were not (control group). In the OAB group, 77 patients were taking dementia medications, and 2,557 were not. The median age (interquartile range) of the dementia cases (n=77) and non-dementia cases (n=2,557) in the OAB group was 80 (70–80) and 70 (60–75), respectively (Table 1). In the control group, 2,390 patients were taking dementia medications, and 167,934 patients were not. The ORs and 95% CIs for drug-induced dementia due to anticholinergic medication use were calculated for 2,634 patients in the OAB medication group and 170,324 patients in the non-dementia medication group. The results showed a significant OR difference, 2.12 (95% CI, 1.66–2.67), indicating an increased risk of drug-induced dementia with OAB medication.
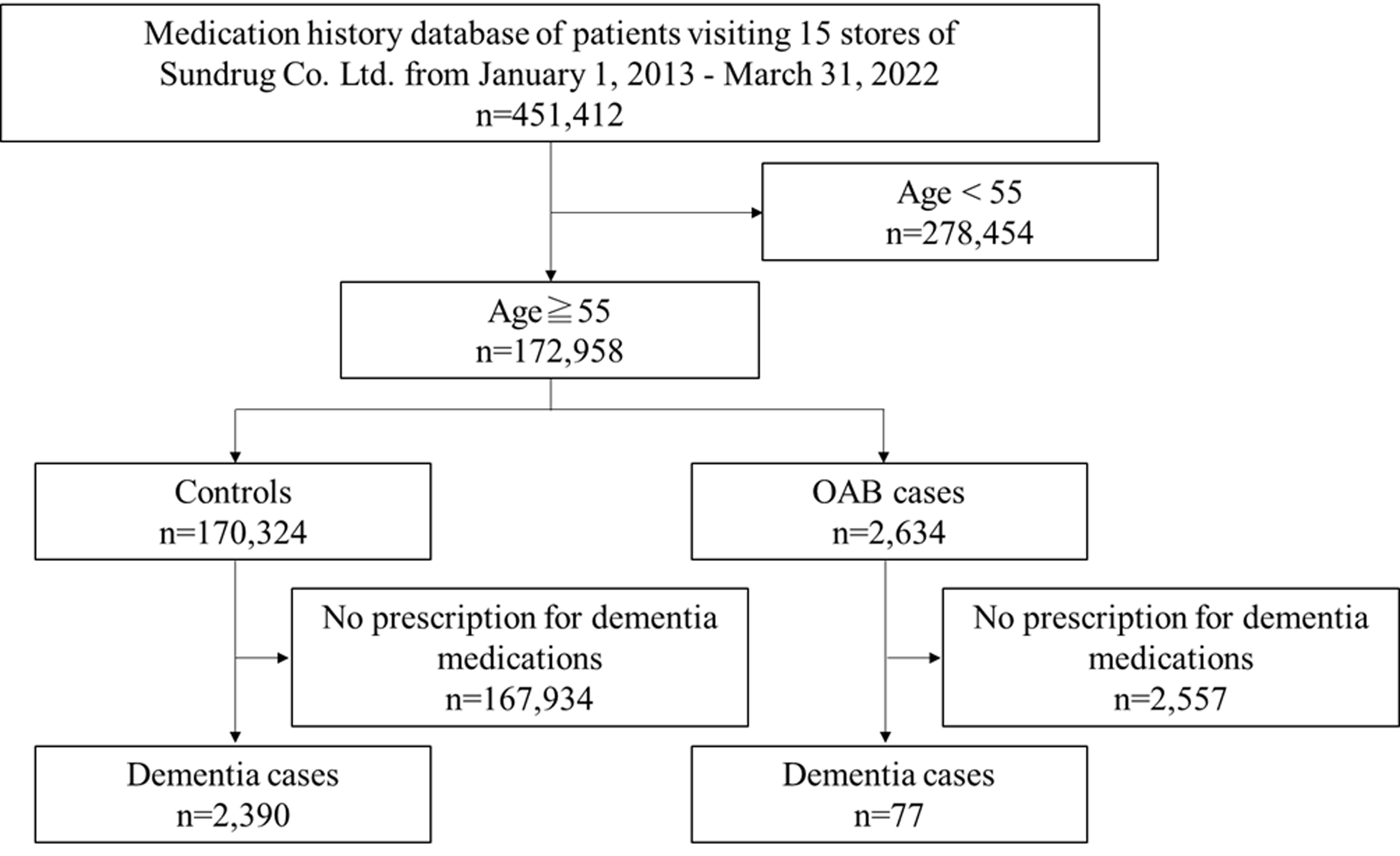
Flow diagram of study participants. OAB: overactive bladder.
The results of this study were compared with those of previously reported large-scale clinical trials (Table 2). Gray et al. [10] evaluated the risk of developing dementia with anticholinergic drugs in a prospective study. They reported an adjusted hazard ratio of 1.54 (95% CI, 1.21–1.96) for cumulative use versus no use of anticholinergic drugs for total standardized daily doses (TSDDs) >1,095. A within-cohort case-control study conducted by Coupland et al. reported that the adjusted hazard ratio for the cumulative use of bladder antimuscarinics was 1.65 (95% CI, 1.56–1.75) for TSDDs >1,095 [11]. Furthermore, Malcher et al. reported the risk of developing dementia with anticholinergic drugs used for OAB and an adjusted OR of 1.48 for >365 cumulative defined daily doses (cDDDs) over the exposure period [12]. These results suggest that long-term use of anticholinergic drugs increases the risk of developing drug-induced dementia, and this risk increases with increasing exposure to anticholinergic drugs [10,11,12].
| References | Indicator used for ascertainment of dementia | TSDDs or cDDDs of anticholinergic drugs | Relative risk (95% CI) |
|---|---|---|---|
| Gray et al. 2015 [10] | DSM-IV and NINCDS-ADRDA criteria | >1,095 TSDDs | 1.54 (1.21–1.96) |
| Coupland et al. 2019 [11] | Diagnosis of dementia in clinical records | >1,095 TSDDs | 1.65 (1.56–1.75) |
| Malcher et al. 2022 [12] | Diagnosis of dementia in clinical records | >365 cDDDs | 1.48 (1.22–1.80) |
| This report | Prescription of dementia medication | All | 2.12 (1.66–2.67) |
TSDD: total standardized daily dose; cDDD: cumulative defined daily dose; CI: confidence interval.
In this study, we detected an increase in the OR for the risk of drug-induced dementia, similar to previous reports in which a cohort or nested case-control study was conducted. The numerical values of the risk ratio were equivalent to those in previous reports. This showed that drug-induced risk assessment could be achieved in a retrospective study using medication history information, yielding similar results to those in clinical studies.
This was a pilot study to investigate the feasibility of medication history; therefore, it has some limitations because of its retrospective approach. First, we did not consider the dose and duration of exposure to anticholinergic drugs when analyzing the ORs for the risk of developing dementia. Previous reports have shown that high cumulative anticholinergic use (TSDDs and cDDDs) is associated with an increased risk of dementia [10,11,12]. In the present study, a similar trend toward an increased OR for dementia with increasing duration of medication was observed (data not shown). Further studies on exposure dose should be considered when risk assessment of developing dementia is conducted. Second, we did not adjust for risk factors for dementia other than anticholinergic drug use. The OR for the risk of developing dementia in patients taking OAB medications was higher than previously reported in large clinical studies. This may be because of the lack of adjustment for dementia-related factors. The typical risk factors for dementia include older age, diabetes, obesity, alcohol consumption, and smoking. In this study, the OR was calculated by including these risk factors, which might have resulted in a higher OR. Furthermore, we assumed that the patients’ visit duration represented the entire medication period and did not consider medication adherence. These conditions might have influenced the OR.
The number of patients included in this study was 172,958, which is comparable with those of previous large-scale clinical trials. However, combining different sets of medication histories accumulated in different communities and expanding the usability of medication histories for drug-induced risk assessment has several problems that must be investigated. For example, adjusting for differences in patient composition among communities, assuring data quality, and standardizing the data. Overcoming these problems may provide a new platform for drug-induced risk assessment.
We verified whether medication history could be used for drug-induced risk assessment. By analyzing the medication histories of 172,958 patients visiting a community pharmacy, we found that anticholinergic drugs may increase the risk of developing dementia. The results were equivalent to those of previously reported large-scale clinical trials, suggesting that medication histories are useful for drug-induced risk assessment.
This work was supported by Sundrug Co., Ltd. The sponsor had no role in the study design, the collection, analysis, and interpretation of data, the writing of the report, and the decision to submit the article for publication.
TO received a research grant from Sundrug Co., Ltd., Tokyo, Japan. ST, JY, SM, TY, MI, and EN have no conflict of interest.
We are grateful to Ms. Wakana Watanabe for her technical assistance. We thank Lorraine Law, BSc, from Edanz (https://jp.edanz.com/ac) for editing a draft of the manuscript.