Article ID: 2025-012
Article ID: 2025-012
The effective oral administration of insulin has the potential to transform diabetes management by improving the quality of life for patients who depend on frequent insulin injections. However, oral insulin delivery faces significant obstacles, including degradation by gastrointestinal enzymes, variable pH environments, and physical barriers such as the mucus and epithelial layers, which result in oral bioavailability as low as ≤2%. To address these challenges, numerous strategies have been explored, with polymeric nanoparticles (PNPs) emerging as a promising approach for enhancing insulin bioavailability via oral routes. This review focuses on the applications of PNPs in oral insulin delivery, emphasizing their potential for overcoming physiological barriers and ensuring controlled and sustained insulin release. It highlights the mechanisms through which PNPs facilitate insulin protection, transport, and release in the gastrointestinal tract, as well as their ability to target specific absorption sites. The review also discusses recent advancements in the design and functionalization of PNPs, such as surface modifications, stimuli-responsive properties, and incorporation of absorption enhancers to improve bioavailability and therapeutic outcomes. Despite promising progress, challenges such as large-scale production, stability during storage, and variability in patient response remain. Furthermore, regulatory and safety considerations must be addressed to accelerate clinical translation. This review serves as a comprehensive guide for researchers aiming to develop advanced PNP-based oral insulin delivery systems. By bridging existing knowledge gaps, it provides a foundation for future innovations that could revolutionize the treatment paradigm for diabetes.
Polymeric nanoparticles offer a promising solution for oral insulin delivery by protecting insulin from degradation, enhancing absorption, and enabling controlled release. This review explores their mechanisms, design advancements, and challenges toward clinical translation for improved diabetes management.
Insulin, a protein hormone secreted by pancreatic islet cells, plays a crucial role in regulating blood glucose levels and is widely used for managing diabetes. It is indispensable for treating type 1 diabetes mellitus (T1DM) and is also employed in advanced cases of type 2 diabetes mellitus (T2DM) when other treatments, such as insulin secretagogues or sensitizers, prove ineffective. Diabetes mellitus is a complex metabolic disorder characterized by diminished insulin secretion or action, leading to hyperglycaemia and disruptions in the metabolism of carbohydrates, proteins, and lipids. T1DM primarily requires insulin therapy, while T2DM is initially managed with oral medications but may eventually necessitate insulin administration [1].
Oral administration of insulin offers a minimally invasive alternative to injections, improving patient compliance. However, it faces significant challenges, such as enzymatic degradation in the gastrointestinal (GI) tract, wide pH variations, and barriers like mucus and epithelial layers, which restrict oral bioavailability to less than 2%. Additionally, oral insulin delivery has inherent limitations, including poor pharmacokinetics, short half-life, and potential side effects, which hinder its clinical utility. To overcome these obstacles, novel nanocarrier delivery systems (NDS) have emerged as promising tools. These systems enhance insulin stability, solubility, bioavailability, and loading capacity while mitigating side effects and protecting insulin from enzymatic degradation in the GI tract. By enabling controlled and targeted release, nanocarriers improve therapeutic efficacy. Upon release, insulin is absorbed into the bloodstream via the portal vein, directly influencing hepatic glucose metabolism and reducing blood glucose levels.
Recent advancements in nanocarriers include lipid-based systems (liposomes, micelles), dendrimers, carbon nanotubes (CNTs), inorganic nanoparticles (quantum dots), gold nanoparticles, and polymeric nanoparticles (PNPs). Among these, PNPs have gained significant attention due to their exceptional stability, biocompatibility, cost-effectiveness, and ability to ensure controlled drug release. Their surface functional groups, such as amino and carboxyl groups, facilitate insulin binding through weak interactions (e.g., hydrogen bonding, van der Waals forces) or covalent bonds, protecting insulin until its delivery to the target site. This tailored approach highlights the immense potential of PNPs in revolutionizing oral insulin delivery systems [2].
Nanocarriers represent a sophisticated drug delivery platform designed to release therapeutic agents precisely at the target site through controlled mechanisms such as the breakdown or diffusion of nanoparticles (NPs). These systems are composed of three fundamental components: a physiologically inert nanocarrier, an active pharmaceutical ingredient (API), and a targeting moiety conjugated to the surface of the carrier. Unlike traditional drug delivery systems (TDDS), which rely on systemic distribution with low specificity and are often associated with adverse effects, nanocarrier systems provide improved drug stability, enhanced selectivity, and reduced toxicity. Furthermore, intelligent drug delivery systems (IDDS) enable precise and sustained therapeutic effects by addressing critical challenges such as solubility, bioavailability, and systemic toxicity, ensuring drug concentrations are maintained within the therapeutic range while also enabling “on-demand” or “programmed” release. The effectiveness of nanocarriers is largely determined by their drug loading capacity, defined as the mass of drug encapsulated per unit mass of nanoparticles, and drugs can either be incorporated during NP fabrication or adsorbed post-manufacture. Critical factors such as nanoparticle structure, surface charge, and size significantly influence their ability to traverse biological barriers like the gastrointestinal epithelium, with optimized designs improving drug bioavailability and absorption, making them particularly advantageous for insulin delivery. Targeted drug release is regulated by various triggers, including pH, solubility, temperature, diffusion through the nanoparticle matrix, and nanocarrier degradation, with stimuli-responsive nanocarriers representing a cutting-edge approach. These nanocarriers, first conceptualized by Takagi et al. [3] in 1990, dynamically respond to environmental changes such as pH, temperature, light, or glucose concentration, facilitating controlled drug release. For instance, Mahobia et al. [4] developed glucose- and temperature-sensitive nanoparticles for insulin delivery, showing increased insulin release at elevated temperatures (18–37°C); however, precise temperature control in vivo remains challenging, as higher temperatures can lead to insulin degradation. pH-responsive nanocarriers, using ionizable polymers like polyacids or polybases, address the pH variability within the gastrointestinal tract (ranging from acidic pH 1.2–3.0 in the stomach to mildly alkaline pH 7.5–8.0 in the intestines) by stabilizing acid-labile drugs such as insulin in harsh gastric conditions and facilitating controlled release in the intestines. For example, chitosan-based nanoparticles (ChNPs) have shown significant potential, as demonstrated by Asal et al. [5], where ChAuNP/PLGA systems achieved insulin retention of 96 ± 0.08% in acidic conditions while enabling prolonged release at higher pH levels. Similarly, Mukhopadhyay et al. [6] developed chitosan-alginate core-shell NPs capable of encapsulating ~85% insulin, with in vitro studies revealing minimal insulin release in stomach-mimicking conditions and extended release in the intestines, resulting in an 8.11% increase in insulin bioavailability in vivo without evidence of systemic toxicity. Moreover, advanced ligand-switchable NPs, such as Pep/Gal-PNPs, extend their ligands in acidic environments to enhance intestinal absorption and expose liver-specific ligands at physiological pH for targeted delivery. In addition to pH responsiveness, dual-responsive systems sensitive to both pH and glucose fluctuations have emerged as a promising solution for oral insulin delivery, particularly for individuals with Type 1 diabetes mellitus (T1DM). These systems either incorporate glucose-sensitive materials such as glucose oxidase (GOx), concanavalin-A, or phenylboronic acid-based polymers or utilize nanocarriers with intrinsic glucose sensitivity. For instance, Jamwal et al. [11] developed a dextran-based glucose- and pH-responsive system in which GOx catalyzed the formation of gluconic acid, triggering insulin release via Schiff base degradation. Xu et al. [35] created ConA-KGM nanoparticles that released insulin in response to glucose concentrations, while Yang Y et al. [8] engineered a polymeric nanoparticle platform capable of extending the therapeutic effect of insulin to 16 hr in Type 1 diabetic mice, significantly reducing the frequency of administration to once daily. Similarly, Benyettou et al. [36] fabricated imine-linked covalent organic frameworks (nCOFs) with a bioavailability of 24.1%, demonstrating minimal insulin release at pH 2.0 but achieving 100% release within 7.5 hr under hyperglycemic conditions, showcasing the potential of dual-responsive nanocarriers for precise and efficient oral insulin delivery (Table1).
| Type of nano-carrier | Size range | Encapsulation efficiency | Load capacity | Zeta potential | Applications in insulin delivery |
|---|---|---|---|---|---|
| Polymeric nanoparticles (PNPs) | 100–500 nm | High (70–90%) | Moderate to High | +20 to −30 mV | Oral insulin delivery, protection from gastrointestinal tract (GIT) enzymes, improved bioavailability |
| Liposomes | 50–1,000 nm | Moderate to High (60–85%) | Low to Moderate | +10 to −30 mV | Insulin encapsulation, controlled release, protection from degradation |
| Solid lipid nanoparticles (SLNs) | 50–1,000 nm | Moderate (60–80%) | Moderate | +15 to −20 mV | Oral insulin delivery, controlled release, and protection from stomach acidity. |
| Nanostructured lipid carriers (NLCs) | 50–1,000 nm | High (70–90%) | Moderate to High | +10 to −25 mV | Oral insulin delivery, enhanced stability, reduced risk of degradation. |
| Polymeric micelles | 10–100 nm | High (75–95%) | Low to Moderate | +10 to −25 mV | Oral insulin delivery, improved solubility, targeted release at specific GIT sites. |
| Dendrimers | 1–10 nm | High (80–95%) | Low | +30 to −50 mV | Insulin delivery, encapsulation for targeted release, improvement of insulin stability. |
| Hydrogels (smart polymers) | 100–1,000 nm | Moderate (60–80%) | High | +10 to −40 mV | Controlled insulin release, protection from acidic pH, site-specific delivery. |
| Chitosan nanoparticles | 100–500 nm | High (75–90%) | Moderate to High | +20 to +40 mV | Oral insulin delivery, enhancement of insulin stability and absorption in GIT. |
| Polymeric microspheres | 100–1,000 nm | Moderate (50–70%) | High | +10 to −20 mV | Controlled and sustained release of insulin, reduced toxicity. |
| Nanocapsules | 10–1,000 nm | High (80–95%) | Moderate | +20 to −30 mV | Insulin delivery, controlled release, targeting specific regions of the GIT. |
Polymeric nanoparticles (PNPs) are emerging as highly promising oral drug delivery systems due to their unique ability to navigate various physiological barriers and their versatile size range, typically between 100 to 500 nm, which facilitates efficient absorption in the gastrointestinal tract (GIT). These nanoparticles can be categorized into two major types: natural polymers and synthetic porous polymers, both of which hold considerable potential for enhancing the oral bioavailability of insulin. Insulin is notoriously difficult to deliver orally because it is vulnerable to enzymatic degradation, variable pH conditions, and absorption obstacles within the GIT. Natural polymers, including polysaccharides, proteins, peptides, and nucleic acids, have been identified as particularly suitable for developing PNP-based oral insulin delivery systems. These biopolymers possess vital attributes such as biodegradability, biocompatibility, non-toxicity, and minimal immunogenicity, making them ideal candidates for safe and efficient drug delivery. Notably, the combination of these natural polymers with bioactive compounds derived from plants with hypoglycemic effects, identified in over 800 species, presents an innovative approach to enhance the therapeutic impact of insulin delivery. This synergy results in dual-functional nanocarriers that not only optimize insulin delivery but also offer additional benefits for blood glucose regulation. Among the natural polymers, polysaccharides such as chitosan, pectin, alginate, glucan, hyaluronic acid (HA), starch, and cellulose are gaining significant attention due to their biocompatibility and biodegradability, essential for developing insulin delivery systems. Chitosan, for example, derived from chitin, has demonstrated the ability to form nanoparticles that effectively encapsulate insulin, offering protection against enzymatic degradation in the stomach while improving the release profile in the GIT. Starch, a widely used polysaccharide composed of amylose and amylopectin, holds promise for drug delivery; however, its high digestibility, rapid enzymatic breakdown, and high glycemic index limit its effectiveness in insulin delivery. To address these drawbacks, modified starch derivatives such as carboxymethyl starch and cyclodextrins have been introduced. These modifications enhance the stability of starch nanoparticles, provide controlled release of insulin, and improve the solubility and bioavailability of hydrophobic drugs. Additionally, proteins derived from sources such as maize, keratin, soy, whey, and casein are being explored for their ability to form protein-based nanocarriers. These systems, including albumin and gelatin, offer inherent biodegradability and biocompatibility, along with ease of modification for functionalization, which aids in encapsulating and stabilizing insulin while ensuring its sustained release in the GIT. The use of proteins also allows for modifications to improve stability under acidic conditions and enzymatic degradation typical of the stomach and small intestine. Despite progress, only two oral insulin systems, ORMD-0801 and HDV-I, have been approved by the U.S. FDA. However, both systems have faced setbacks in clinical trials due to concerns over toxicity, instability, and adverse effects, preventing their market release. These challenges underscore the need for continued research into optimizing the physicochemical properties of nanoparticle carriers, including their size, surface charge, and release kinetics, to ensure their clinical viability. One promising avenue for overcoming these limitations is the development of smart polymeric hydrogels capable of responding to environmental conditions in the GIT. These hydrogels are designed to protect insulin from acidic gastric conditions and enzymatic degradation while facilitating controlled release as they transit to more neutral pH environments in the intestines. Yang et al. [7] demonstrated the effectiveness of a carboxymethyl chitosan hydrogel modified with carboxymethyl cyclodextrin (CMCD)-grafted microparticles, which successfully protected insulin in the gastric environment and ensured its slow release in the GIT. [8] The hydrogels were synthesized using the EDC/NHS coupling method to covalently link carboxymethyl chitosan with CMCD, producing a biocompatible and biodegradable system that not only retained insulin in acidic conditions but also facilitated controlled release in the intestine. [9] This type of “smart” hydrogel system marks a significant step forward in the development of oral insulin delivery, as it mimics the body’s natural insulin release patterns, offering the potential for more effective and sustained insulin therapy. [10] Further research and optimization are necessary to address the remaining challenges and unlock the full potential of PNPs for oral insulin delivery [11] (Figs. 1, 2).
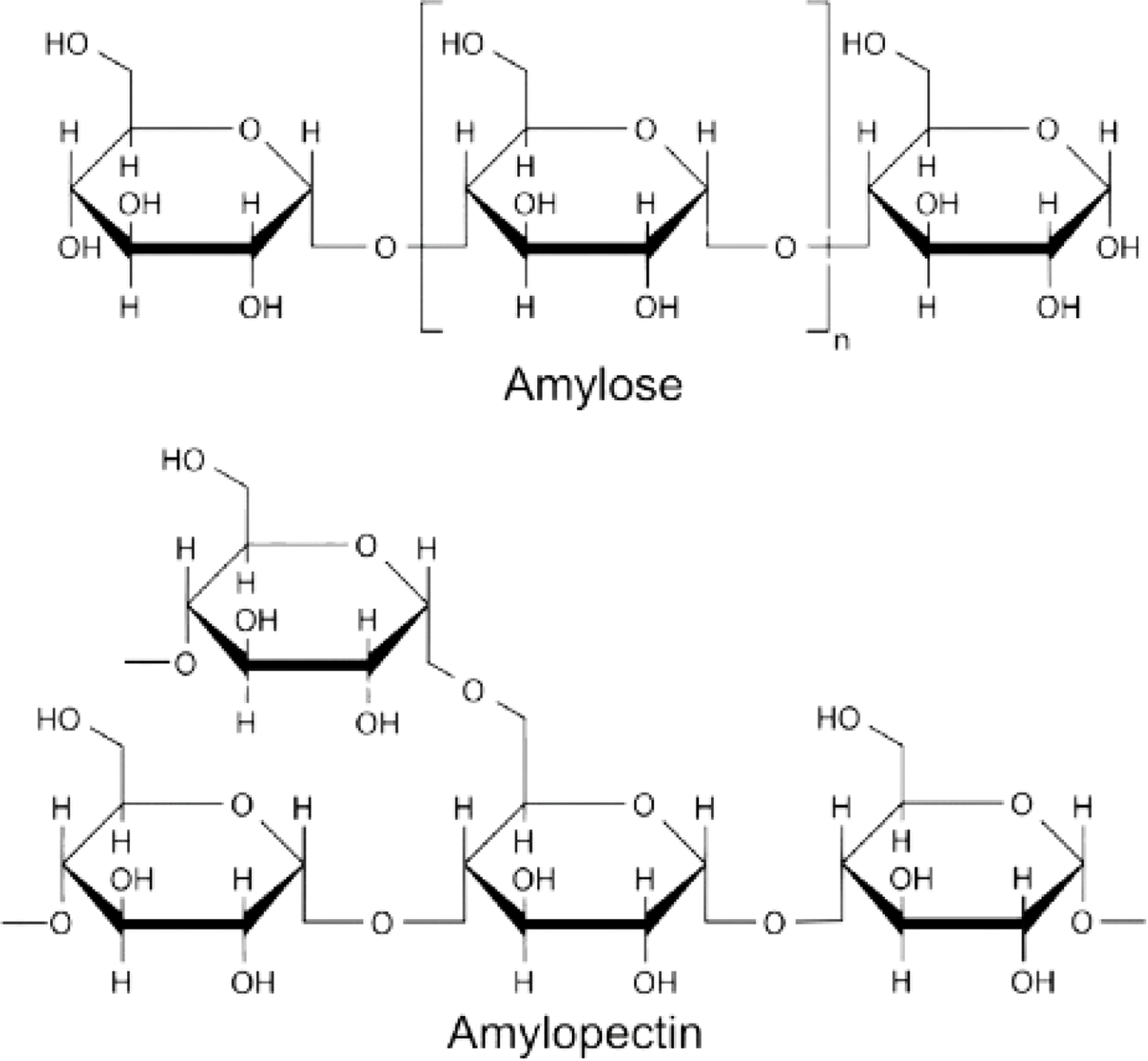
Structural components of Starch.

Structural components of Chitosan.
Synthetic porous polymers (SPPs) offer distinct advantages over natural polymers and other material types, primarily due to their versatile design, allowing for the production of tailored materials to meet specific therapeutic needs and patient demands. These polymers are engineered to address various medical applications, particularly in drug delivery systems. Notable examples include activated charcoal, silica nanoparticles (SNs), porous coordination polymers (PCPs), porous organic polymers (POPs), and others. Among these, POPs—comprising subtypes like hyper-cross-linked polymers (HCPs), polymers with intrinsic microporosity (PIMs), conjugated microporous polymers (CMPs), porous aromatic frameworks (PAFs), and covalent organic frameworks (COFs)—stand out due to their distinct pore structures, tunable pore sizes, customizable surface properties, and structural flexibility, making them suitable for various delivery needs. In the realm of oral insulin delivery, SPPs, including metal-organic frameworks (MOFs) and COFs, have shown promise in enhancing bioavailability and enabling controlled, delayed insulin release, thereby overcoming the gastrointestinal barriers that hinder oral insulin administration. These materials protect insulin from degradation and facilitate its controlled release, a crucial advantage in oral insulin formulations. Activated charcoal, known for its adsorptive properties, has been explored for treating metabolic disorders like obesity and insulin resistance. Research indicates that when combined with an acidic environment, such as a high-fat diet model, activated charcoal reduces obesity and improves insulin sensitivity in a dose-dependent manner [12]. However, its alkaline nature may interfere with gastric acidity, diminishing its protective effect against pepsin, and its powder form may affect the gastrointestinal tract, requiring further optimization for insulin delivery. Mesoporous silica nanoparticles (MSNs) have been investigated for oral insulin delivery due to their biocompatibility, high encapsulation efficiency, and controlled release. Studies have shown that silica nanoparticles can encapsulate insulin, protecting it from gastric degradation and ensuring efficient transport to the intestine. Despite their promise, silica nanoparticles face challenges such as poor epithelial membrane penetration and complex synthesis processes, limiting their scalability [13]. Lastly, PCPs, including MOFs, are composed of metal ions or clusters coordinated with organic ligands, and are biodegradable, offering significant advantages over traditional inorganic nanostructures [14]. With over 20,000 types of MOFs developed, their biodegradability and versatility make them ideal for oral insulin delivery, where they provide controlled release and protection against gastrointestinal degradation, thus enhancing insulin stability and bioavailability. These materials represent a significant step forward in the development of effective oral insulin therapies [15].
Metal-Organic Frameworks (MOFs) are typically synthesized in a single-step process that involves metal salts, organic ligands, and solvents, usually under moderate to low temperature conditions, which facilitates the formation of porous crystalline structures. Several advanced techniques have been developed to optimize MOF synthesis, including solvothermal synthesis, reverse micro-emulsion, template-based approaches, as well as microwave-assisted, direct precipitation, and sonochemical methods. These innovative methods provide improved control over synthesis conditions, enhancing the uniformity, crystallinity, and pore structure of MOFs, with microwave-assisted synthesis and sonochemical methods standing out for their rapid reaction times and high yields, making them ideal for large-scale production. To tailor MOFs for specific applications such as drug delivery, gas storage, catalysis, and sensing, functionalization plays a crucial role in modifying their physical, chemical, and structural properties. Functionalization can be divided into two main approaches: pre-functionalization and post-synthetic modification (PSM). Pre-functionalization involves incorporating functional groups into the MOF framework before synthesis, typically by modifying the organic ligands or metal nodes. This approach offers precise control over the type and density of functional groups, allowing for targeted material design, but is limited by the availability of compatible functionalized ligands and the complexity of introducing multiple groups. On the other hand, PSM introduces functional groups to an already synthesized MOF, providing greater flexibility and control over modifications such as covalent functionalization, coordination-based modifications, surface functionalization, ion exchange, and polymerization post-synthesis. This method is advantageous in enhancing the material’s stability, catalytic properties, or adsorption capabilities, and is particularly useful when pre-functionalization is not feasible. However, PSM requires careful optimization of conditions to avoid incompatibility issues, as certain MOFs may be sensitive to the reaction conditions. Despite these challenges, PSM allows for precise fine-tuning of the material’s properties, offering significant advantages in tailoring MOFs for diverse applications.
Metal-organic frameworks (MOFs) are emerging as highly promising candidates for drug delivery systems (DDS), offering unique advantages over conventional materials. Their exceptional properties, such as large surface areas, high porosity, and tuneable structures, enhance the efficiency of drug loading. Additionally, MOFs exhibit excellent biocompatibility, water solubility, and biodegradability, which improve the bioavailability and therapeutic effectiveness of drugs within the body. These materials allow for a variety of interactions, including hydrogen bonding, Van der Waals forces, electrostatic interactions, and coordination bonds, to encapsulate or chemically bond drugs, biomolecules, and guest molecules. This versatility has enabled the encapsulation of a wide range of drugs such as doxorubicin (DOX), 5-fluorouracil (5-Fu), β-estradiol, and insulin. Recent advances in MOF-based systems have focused on the oral delivery of insulin, a challenging but highly beneficial therapeutic approach for diabetes management. Traditional insulin delivery methods, including injections, are inconvenient and invasive, making the development of an effective oral insulin delivery system highly desirable. Researchers have developed various MOF-based strategies to overcome the barriers to oral insulin bioavailability, such as enzymatic degradation in the gastrointestinal (GI) tract, pH variability, and the mucosal barrier.
For example, Chen et al. [22] synthesized a Zr-based MOF (NU-1000) that demonstrated excellent acid resistance and efficient insulin loading. The pores of NU-1000 interact favourably with insulin, and the material undergoes disintegration in the presence of phosphate ions, mimicking blood conditions. This system was capable of loading approximately 40% insulin by weight, with stable insulin release observed under physiological pH (7.0 in PBS) within 30 mins. In vivo studies indicated that the NU-1000 system significantly enhanced insulin bioavailability when administered orally in a mouse model. Similarly, Zhou et al. [16] developed a polymer-coated MOF system using MIL-100, which encapsulated both insulin and sodium dodecyl sulfate (SDS). The polymer coating, made of methoxy poly (ethylene glycol)-block poly (l-lactide), protected the MOF from degradation in the GI environment, while SDS facilitated enhanced permeability across the intestinal membrane. The MIL-100 nanoparticles demonstrated excellent cellular uptake in Caco-2 monolayer cultures, and in vivo studies showed that the system achieved a maximal plasma insulin level (50 mIU/ml) at 4 hr, maintaining higher insulin levels for up to 8 hr in a BALB/c mouse model of type I diabetes. Notably, this system exhibited a more gradual and prolonged reduction in blood glucose levels compared to subcutaneous insulin injections, with insulin accumulating in the liver and mimicking endogenous insulin circulation patterns. Rohra et al. [37] took a novel approach by utilizing microfluidic techniques to synthesize a glucose-responsive insulin delivery system using the metal-organic framework (MOF) Ins-AuNPZIF-8. The insulin loading capacity was determined to be 77% and 88% using size exclusion chromatography and high-performance liquid chromatography, respectively. The system was capable of releasing insulin in response to elevated glucose levels, offering a targeted and controlled release mechanism. This study marks the first application of a SAR micromixer in the synthesis of MOFs, particularly ZIF-8, to encapsulate insulin and gold nanoparticles (AuNPs), opening new avenues for glucose-triggered insulin delivery. Additionally, Zou et al. [16] developed an acid-resistant MOF (UiO-68-NH2) encapsulating insulin, which was coated with transferrin proteins to enhance targeting and facilitate intestinal absorption. The transferrin-coated nanoparticles effectively crossed the intestinal epithelium, releasing insulin under physiological conditions, which led to a significant hypoglycemic response. This formulation demonstrated an oral bioavailability of 29.6%, showing the potential of MOFs to protect sensitive proteins from the harsh GI environment and to improve the systemic delivery of macromolecules.
These recent advancements in MOF-based insulin delivery systems highlight their potential to revolutionize oral insulin administration. MOFs offer significant advantages in protecting insulin from degradation, enhancing its absorption, and ensuring controlled release, making them a promising platform for the development of next-generation oral drug delivery systems. Continued research is needed to optimize these systems for large-scale production, address regulatory challenges, and ensure safety for clinical use. [17,18,19]
Porous Organic Polymers (POPs) have emerged as highly promising materials for advancing oral insulin delivery systems, offering significant advantages over conventional porous materials like activated charcoal, silica, and porous carbon polymers (PCPs). These POPs exhibit enhanced surface areas and tightly controlled size distributions, making them ideal candidates for drug delivery, particularly for insulin administration. The interaction between the drug and the carrier in POPs occurs through relatively weak forces, including van der Waals interactions, hydrogen bonds, and other non-covalent interactions, which allow for efficient encapsulation and release of insulin. POPs comprise several specialized polymers, such as hyper-cross-linked polymers (HCPs), polymers with intrinsic microporosity (PIMs), conjugated microporous polymers (CMPs), porous aromatic frameworks (PAFs), and covalent organic frameworks (COFs), each having distinct structural properties. Among these, COFs are particularly noteworthy for their crystalline structure, while the others, including HCPs, PIMs, CMPs, and PAFs, remain amorphous. COFs, due to their ordered, crystalline nature, offer unique benefits for controlled and sustained insulin release, making them highly suitable for oral delivery applications. COFs are crystalline, porous organic polymers composed of light elements such as carbon, hydrogen, oxygen, nitrogen, and boron, and are synthesized through covalent bonds formed via reversible reactions. This gives COFs outstanding characteristics, including low density, high surface area, customizable pore sizes, and well-ordered internal structures, providing high modifiability and versatility for various applications, such as drug encapsulation, release, biosensing, and catalysis. The ordered and customizable nature of COFs allows for fine-tuning to optimize drug encapsulation and release profiles, making them particularly beneficial in the context of oral insulin delivery. COFs can be categorized into two-dimensional (2D) and three-dimensional (3D) structures, each with distinct properties. 2D COFs often feature monomers with geometries such as C1, C2, C3, C4, and C6, enabling the formation of hexagonal, rhombic, and kagome-type frameworks. These are commonly used in boroxine- and triazine-linked COFs, which have proven effective for drug encapsulation. On the other hand, 3D COFs are typically formed using monomers with Td symmetry, allowing for the construction of more complex, interpenetrated networks. The design of COFs heavily relies on topological graphs that define the geometry of the monomers and their interaction patterns. For 2D COFs, self-condensation of monomers with specific geometries, such as [C2 + C2 + C2] or [C3 + C2], results in hexagonal frameworks. In contrast, 3D COFs require more intricate topological configurations, incorporating monomers with Td symmetry to create interconnected structures. These networks may exhibit varying degrees of interpenetration, which impacts their stability and drug release properties. The adaptability of COFs allows for a broad range of topologies, including dia, pts, ctn, bor, srs, and helical structures, offering distinct advantages for applications in drug encapsulation, release kinetics, and biocompatibility, particularly for insulin delivery [20, 21]. Synthesis of COFs typically involves various methods, each with its own set of advantages and challenges. The solventothermal method is the most widely used, although it requires long reaction times, ranging from 2 to 7 days. Other methods, such as microwave synthesis, interface synthesis, mechanical grinding, sonochemical synthesis, and room-temperature synthesis, have been explored to improve synthesis efficiency and reduce time. Room-temperature synthesis, for example, has been successfully demonstrated using Schiff-base reactions, allowing for rapid production without the need for high temperatures or prolonged reaction times. Researchers have also developed methods to further shorten COF synthesis times, such as using ionic liquids, which enable high-quality COF production within mins under ambient conditions, making large-scale production more feasible. This is critical for commercializing COFs for oral insulin delivery applications. The functionality of COFs is largely determined by their pore structures and the types of functional groups incorporated into their frameworks. There are three main approaches for introducing functional groups into COFs: bottom-up incorporation, in situ functionalization, and post-modification. In the bottom-up approach, functional moieties are incorporated into the monomers before COF synthesis, ensuring a uniform distribution of active sites across the COF framework. While this approach allows for precise control over active site density, it can hinder crystallization when sterically bulky functional groups are used. In situ functionalization occurs during the COF condensation process, where specific functional groups are incorporated at designated locations. Although this method is simpler than bottom-up incorporation, it limits the ability to control the location and density of the functional groups, which can affect the performance of COFs in drug delivery. The post-modification method involves introducing functional groups into the COF framework after its initial synthesis. While this approach provides greater flexibility in adding a wide range of functional groups, it can also disrupt the COF’s structure and porosity, making the outcome less predictable. Each of these functionalization strategies plays a vital role in optimizing COFs for specific applications, such as improving drug loading capacity, controlling release profiles, and targeting specific sites in the gastrointestinal tract for enhanced insulin absorption and therapeutic effect [23, 24, 29].
Covalent organic frameworks (COFs) have garnered significant attention in drug delivery systems due to their exceptional characteristics, such as high surface area, excellent crystallinity, porosity, and biocompatibility. These attributes allow COFs to achieve high drug loading capacities and efficient drug delivery. The most commonly employed COFs for drug delivery include those with imine, imide, hydrazone, and triazine linkages, though other types such as phenazine and olefin-linked COFs have also been explored. The high nitrogen content in these structures, with available lone pair electrons, enables the loading of drugs via hydrogen bonding or π−π interactions, significantly enhancing their drug-loading efficiency.
One of the pioneering studies in COF-based drug delivery was conducted by Fang et al. [38] in 2015, who developed polyimide-based COFs (PI-COFs) with large, uniform pores and high stability. They successfully demonstrated the loading and controlled release of ibuprofen (IBU), marking the first use of COFs in drug delivery. This breakthrough opened the door for further innovations in COF-based nanodrug delivery systems (NDDSs). In 2016, Bai et al. [25] utilized two nanoscale porous COFs to load the anticancer drug 5-fluorouracil (5-FU), demonstrating excellent aqueous dispersibility, high drug loading capacity (up to 30 wt%), and complete in vitro drug release. Similarly, Vyas et al. [26] synthesized triazine-linked COFs (TTICOF) for the delivery of quercetin, showcasing their potential as nanocarriers. In subsequent years, COFs have been modified for various applications, particularly in cancer therapy. For example, Mitra et al. [27] in 2017 developed folate-conjugated COFs for targeted delivery of 5-FU to breast cancer cells, while Zhang et al. [28] in 2018 created a polymer-COF nanocomposite for enhanced delivery of doxorubicin (DOX) to tumors. Further advancements were made in 2020 by Liu et al. [30], who developed redox-responsive nanoscale COFs for doxorubicin delivery, achieving a high drug-loading content of 21%. In 2021, Anbazhagan et al. [31] synthesized a thioether-terminated triazole-bridged COF (TCOF) for pH- and glutathione-sensitive drug delivery, demonstrating a 70–80% drug release within 72 hr.
Despite the extensive research on COFs for drug delivery, their application in oral insulin delivery has only recently emerged. In 2021, Benyettou et al. [36] introduced an imine-based COF, TTA-DFP-nCOF, as a novel carrier for oral insulin delivery. This COF, particularly due to its stability in acidic environments, was chosen for its superior insulin-loading capacity (65 wt%) and ability to protect insulin under harsh gastrointestinal conditions. The COF’s pore size (around 1.7 nm) is smaller than the molecular size of insulin (2.5–3 nm), suggesting that insulin is intercalated within the COF layers rather than occupying the pores. This unique structural feature allows the efficient protection and controlled release of insulin, with improved bioavailability (24.1%) observed in animal models. When administered orally, TTA-DFP-nCOF-encapsulated insulin was rapidly absorbed by the intestinal epithelium and transported to the liver via the portal vein, effectively maintaining glucose homeostasis. Notably, no significant damage was observed in the liver or kidneys of the insulin-treated rats, suggesting the biocompatibility and non-toxic nature of COFs. In fact, these COF-based systems showed promising improvements in liver and kidney functions, underscoring the potential of COFs for safe and effective oral insulin delivery [32,33,34].
Polymeric nanoparticles (PNPs) present considerable promise for oral insulin delivery, with ongoing advancements in both natural and synthetic polymer nanocarriers. However, challenges persist in demonstrating long-term efficacy, particularly in larger animal models and humans. Although natural small-molecular polymers have been frequently used, their pore size control remains a limitation. Future research must focus on developing synthetic porous polymer nanoparticles with better control over pore size and structural integrity to optimize insulin delivery. Before transitioning to clinical applications, oral insulin formulations must be optimized for key parameters such as encapsulation efficiency, loading capacity, gastrointestinal stability, bioavailability, and intestinal permeation. Despite promising results in pre-clinical studies, the clinical realization of oral insulin remains distant. Moreover, the bioavailability and stability of insulin in the gastrointestinal tract continue to present significant barriers. Metal-organic frameworks (MOFs) and covalent organic frameworks (COFs) are emerging as novel candidates due to their high drug loading capacity and bioavailability. COFs, in particular, show potential for optimized insulin delivery through precise manipulation of pore sizes and interlayer spaces. Future research should focus on fine-tuning these characteristics, allowing for effective insulin loading (2.5–3.0 nm) within COF structures, thereby enhancing oral insulin delivery efficiency. As research progresses, these frameworks could play a pivotal role in transforming oral insulin therapy for diabetes management.
The Authors declare no conflict of interests.