2020 年 2 巻 1 号 p. 3-12
2020 年 2 巻 1 号 p. 3-12
BACKGROUND
In atrial-fibrillation (AF)-related acute ischemic stroke (AIS), the optimal timing for starting or resuming non-vitamin K antagonist oral anticoagulants (NOAC) remains unknown. We aimed to determine the optimal timing of NOAC initiation for AF-related AIS using the Japanese Diagnosis Procedure Combination inpatient database.
METHODS
We retrospectively collected data of adult inpatients who were admitted with a diagnosis of AF-related AIS from July 2010 to March 2016. Patients were divided into tertiles of hospital days for initiating NOAC (early, delayed, and late groups). Incidence of hemorrhagic events or NOAC discontinuation was the primary outcome. Secondary outcome included in-hospital death, any hemorrhagic event, thrombotic events, and deterioration of modified Rankin scale from admission to discharge. Logistic regression analyses were performed to compare the primary and secondary outcomes among the three groups with adjustments for patient backgrounds using inverse probability of treatment weighting by propensity score analysis.
RESULTS
We identified 55,289 eligible patients, including 17,810 in the early group (3–5 days), 18,473 in the delayed group (6–10 days), and 19,006 in the late group (≥11 days). Logistic regression analyses indicated the odds ratio for the primary outcome in the late group was not significant (adjusted odds ratio, 1.08; 95% confidence interval, 1.0–1.16).
CONCLUSIONS
NOAC initiation within 3–5 days was safer and associated with the lower proportions of the secondary outcomes in patients with AF-related AIS.
In acute ischemic stroke (AIS), the prevalence of atrial fibrillation (AF) is increasing [1], and AF is associated with increased in-hospital mortality [2]. AIS followed by recurrence of stroke or cerebral hemorrhage confers a high risk of devastating outcomes including death. Thus, establishing a strategy for AF-related AIS is a challenge.
An adequate strategy for AF-related AIS remains undetermined. Previous studies demonstrated effectiveness or safety of anticoagulation agents including warfarin [3, 4]. A meta-analysis showed that anticoagulant use for acute cardioembolic stroke increased symptomatic cerebral hemorrhage [5].
The safety and effectiveness of the early initiation of non-vitamin K antagonist oral anticoagulants (NOAC) is attracting attention; however, studies on the optimal timing and outcomes of NOAC administration are limited. Reported prospective or randomized controlled studies included only patients with either transient ischemic attack or AF-related mild ischemic stroke [6, 7]. Because of a lack of evidence covering various severities of AF-related AIS, expert recommendations from the European Society of Cardiology or the American Heart Association/American Stroke Association have not been made [8, 9]. The optimal timing of NOAC administration for patients with AF-related AIS of differing severity, especially moderate or severe AF-related AIS, is yet to be elucidated.
The aim of the present study was to investigate the outcomes of the early initiation of NOAC among patients with mild-to-severe AF-related AIS, in a retrospective observational study using a national inpatient database in Japan.
We used the Japanese Diagnosis Procedure Combination inpatient database [10]. The database contains information of inpatients from over 1,000 hospitals across Japan, with approximately 7,000,000 patients per year. The database includes approximately 50% of all Japanese inpatients in the acute phase. The database contains administrative claims and discharge data including the following information: sex and age; body height and weight; date of hospitalization and discharge; urgent or planned admission; diagnoses, comorbidities at admission, and complications during hospitalization, which are classified according to the International Classification of Diseases Tenth Revision (ICD-10) codes and text data in Japanese; procedures; drugs; discharge status including transferred or death; and each hospital identification number. A validation study on the database demonstrated that the sensitivity and specificity of primary diagnoses were 78.9% and 93.2%, respectively [11]. The requirement for informed consent was waived because of the anonymous nature of the data. The present study was approved by the Institutional Review Board of The University of Tokyo. The Board waived informed consent because of the anonymous nature of the data.
PATIENT SELECTIONWe selected patients hospitalized with a diagnosis of ischemic stroke (ICD-10 code of I63) from 01 July 2010 to 31 March 2016 and who received NOAC (dabigatran, rivaroxaban, apixaban, and edoxaban) administration during hospitalization. The exclusion criteria were patients aged less than 18 years, those who were deceased within 2 days of admission, and those who received NOAC within 2 days of admission.
DATA COLLECTION AND PATIENT BACKGROUND CHARACTERISTICSWe examined the following patient background characteristics on admission: age; sex; body mass index (BMI); comorbidities including diabetes mellitus, dyslipidemia, hypertension, heart failure, previous stroke, chronic obstructive pulmonary disease; Japan Coma Scale (JCS); modified Rankin scale (mRS) at admission and discharge; use of antithrombotic drug (aspirin, cilostazol, clopidogrel, ticagrelor, ticlopidine, sarpogrelate, warfarin, unfractionated heparin, LMWH, alteplase, and argatroban) within 2 days of admission; administration of edaravone, ozagrel, and hyperosmolar drugs within 2 days of admission; either administration of histamine-2 receptor antagonists, proton pump inhibitors or vonoprazan within 2 days of admission; blood transfusion (red blood cell transfusion, fresh frozen plasma, and platelet); reversal agents for anticoagulation therapy; administration of tranexamic acid or carbazochrome; hemodialysis or continuous renal replacement therapy within 2 days of admission, mechanical ventilation; or magnetic resonance imaging within 2 days of admission. Unplanned admission, ambulance use, endovascular procedures for stroke, rehabilitation by physical therapist, and admission to stroke care unit or intensive care unit was also identified. Patient age was classified into ≤72 years, 73–81 years, and ≥81 years by tertiles of age. BMI was classified as underweight (<18.5 kg/m2), low-normal weight (18.5–22.99 kg/m2), high-normal weight (23–24.99 kg/m2), overweight (25–29.99 kg/m2), and obese (≥30.0 kg/m2). The CHADs2 score [12] was calculated as a weighted integer score of age and specific comorbidities (diabetes mellitus, hypertension, heart failure, and previous ischemic stroke) subject to ICD-10 codes. Modified Rankin scale consists of integer scores ranged from 0 (no symptoms) to 6 (death). Eligible patients were divided into tertiles of hospital day of NOAC administration (early, delayed, or late group).
OUTCOMESThe primary outcome was incidence proportion of hemorrhagic event after NOAC initiation or NOAC discontinuation. The secondary outcomes were in-hospital death, any hemorrhagic event, thrombotic event, and deterioration of mRS from admission to discharge. Hemorrhagic events after NOAC initiation were identified as follows: receiving craniotomy for hematoma removal; gastrointestinal endoscopic hemostasis and surgical hemostasis for gastrointestinal hemorrhage after NOAC initiation. Any hemorrhagic event was identified as follows: receiving hemostasis procedure (craniotomy for hematoma removal; gastrointestinal endoscopic hemostasis and surgical hemostasis for gastrointestinal hemorrhage); discontinuation of NOAC; blood transfusion; use of reverse agents; and ICD-10 codes of gastrointestinal or intracranial hemorrhage classified as occurrence during hospitalization. Any hemorrhagic event included all events either before or after NOAC initiation because the outcome included events identified by ICD-10 codes, which do not distinguish between before or after NOAC initiation in the database. Any thrombotic event was defined as follows: occurrence of acute myocardial infarction, pulmonary thrombotic embolism, or peripheral embolism during hospitalization. Deterioration of mRS was defined as increasing mRS at discharge by at least 1 compared with mRS at admission. In light of the relevance of mRS deterioration, we checked the proportion of AF patients without mitral valve disease or valve surgery who had mRS of 3 to 5 at discharge for consistency between mRS deterioration and mRS of 3 to 5.
STATISTICAL ANALYSESCharacteristics were examined for all patients who were hospitalized for ischemic stroke. Categorical variables were expressed as the number and proportion. The crude proportions of the outcomes and these confidence intervals were calculated, and then compared among the three groups by Fisher’s exact test with the Bonferroni correction (to allow for multiple testing, the significance level was set at a p value of 0.016). To compare the primary outcome, we conducted an inverse probability of treatment weighted analysis to adjust for measured confounders. Propensity score model for the three groups was established by applying generalized boosted model [13] conditioned on 10000 regression trees among patient background variables, including age, sex, BMI, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, previous ischemic stroke, unplanned admission or urgent admission, JCS at admission, mRS at admission, and drugs and procedures within 2 days of admission (antiplatelet agents; antacid agents; endovascular procedure for stroke; hemodialysis or continuous renal replacement therapy; mechanical ventilation; edaravone; glycerol; ozagrel; unfractionated heparin or LMWH; warfarin; alteplase; argatroban; stroke care unit or intensive care unit admission; or rehabilitation by physical therapist). Standardized differences of less than 0.1 are considered negligible imbalances in baseline characteristics between groups [14]. We confirmed that the standardized differences of all covariates were less than 0.1 after adjustment with inverse probability of treatment weighting based on generalized boosted model (Fig. 1). This calculates an optimally balanced model to obtain the average treatment effect based on regression trees and can be applied to balancing between three or more groups. Logistic regression analyses were conducted to compare the outcomes between the three groups for hospital days before initiating NOAC among all eligible patients.
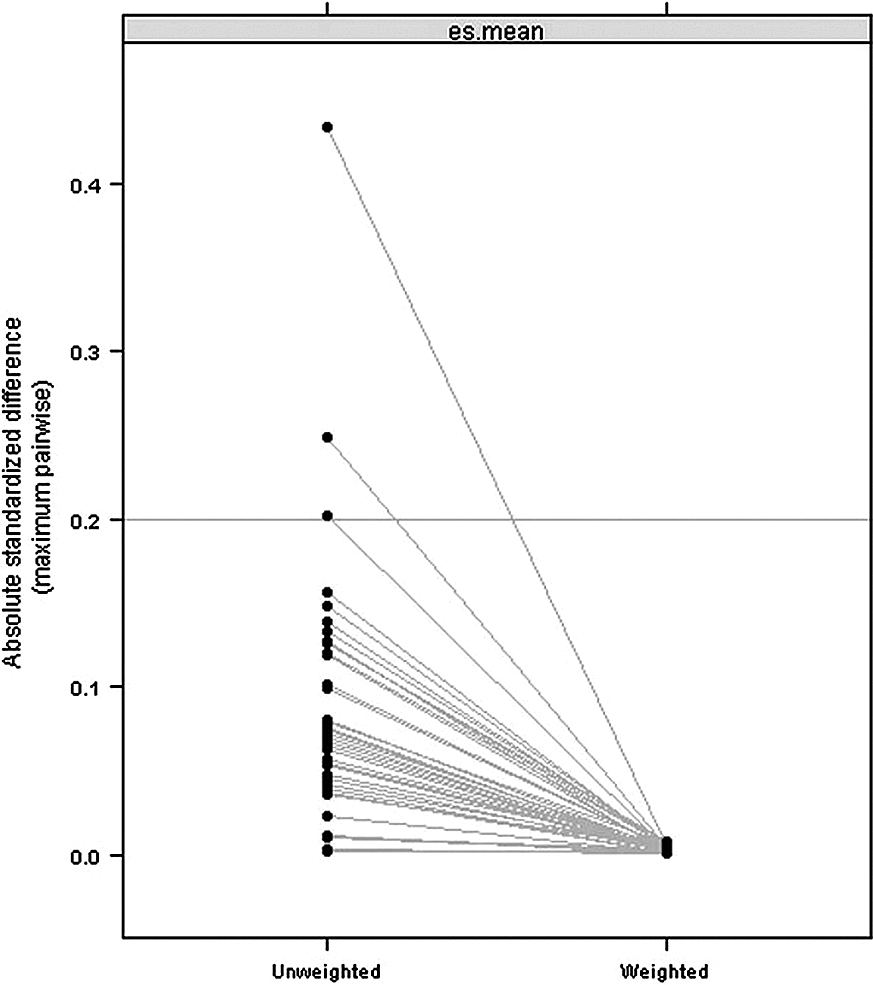
Figure summarized the blance of the covariates by illustrating the absolute standardized difference on pretreatment between before and after inverse probability weighting. The “es” refers to the absolute standardized difference. These are summarized across the pretreatment variables by the mean.
Sensitivity analyses were conducted to be compared with the primary analysis. We conducted multivariable logistic regression analysis including patients who received NOAC within 2 days of hospitalization in the sensitivity analysis. We used the same covariates to those in the main analysis. An additional sensitivity analysis was performed using logistic regression fitted with a generalized boosted model for AF patients without mitral valve diseases or valve surgery. First, we excluded patients with mitral valve heart disease using ICD-10 codes (mitral valve disease: I050, I051, I052, I058, I059, I080, I081, I083, I088, I340, I341, I342, I348, Q232 or Q233; and valve replacement or plasty: Z988; Z952, Z953 or Z954). Second, we identified patients with atrial fibrillation using ICD-10 code I48.x. Third, we excluded patients if NOAC was switched to warfarin. We fitted the multivariable analysis with a generalized estimating equation adjusting for: the timing of NOAC initiation, mRS at admission, interaction term of NOAC initiation with mRS at admission, age, sex, BMI, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, previous ischemic stroke, unplanned admission or urgent admission, JCS at admission, drugs and procedures within 2 days of admission (antiplatelet agents; antacid agents; endovascular procedure for stroke; hemodialysis or continuous renal replacement therapy; mechanical ventilation; edaravone; glycerol; ozagrel; unfractionated heparin or LMWH; warfarin; alteplase; argatroban; stroke care unit or intensive care unit admission; or rehabilitation by physical therapist).
Subgroup analyses calculating odds ratios comparing the timing of NOAC initiation (3–10 days or ≥11 days) for the primary outcome were performed. The subgroup included mRS at admission (0 to 2 or ≥3), age category, sex, history of previous stroke, JCS at admission, antiplatelet use in pretreatment, heparin use in treatment, mechanical ventilation in treatment.
We included missing BMI data (category variable) as a missing category in the analyses because the proportion of missing BMI data accounted for approximately 10%. Covariates and outcomes other than BMI were analyzed by complete case analysis.
All statistical analyses were conducted with Stata software version 15 (StataCorp LP, College Station, TX) and R version 3.0.1. The results were reported with 95% confidence intervals. Statistical significance was set for two-sided p values <0.05.
We identified 91,937 hospitalized patients who received NOAC after hospitalization for AF-related AIS during the study period. The following patients were excluded: (i) 7 patients aged under 18 years on admission; (ii) 143 patients who were deceased within 2 days of admission; (iii) 30,403 patients who received NOAC within 2 days of admission. Subsequently, we restricted patients on first admission. Consequently, 55,289 eligible patients were divided into tertiles by hospital days before initiating NOAC, resulting in 17,810 patients receiving NOAC at 3–5 hospital days (the early group), 18,473 at 6–10 days (the delayed group), and 19,006 after 11 days (the late group).
PATIENT CHARACTERISTICSThe patient characteristics are summarized in Table 1 Among the total patients, approximately 37% were aged over 82 years, 56% were male, and 4.8% had previous stroke history at admission. The hospital day of NOAC administration ranged day 3 to 1,362 and the median was day 7 [interquartile range (IQR): 3, 12]. The median length of hospital stay was 19 [IQR: 12, 34] days for the early group, 23 [IQR: 15, 38] days for the delayed group, and 41 [IQR: 26, 63] days for the late group. The median length of stay in the stroke care unit was 6 [IQR: 4, 10] days for the early group, 7 [IQR: 4, 10] days for the delayed group, and 8 [IQR: 5, 11] days for the late group. The median length of stay in the intensive care unit was 1 [IQR: 1, 4] day for the early group, 2 [IQR: 1, 4] days for the delayed group, and 3 [IQR: 2, 7] days for the late group.
| Early (3–5 days) (N = 17,810) | Delayed (6–10 days) (N = 18,473) | Late (≥11 days) (N = 19,006) | |
|---|---|---|---|
| Age (years), n (%) | |||
| Mean (standard deviation) | 76.8 (10.5) | 76.5 (10.6) | 77.2 (10.6) |
| ≤72 | 5,591 (31.4) | 5,855 (31.7) | 5,454 (28.7) |
| 73–81 | 5,758 (32.3) | 6,044 (32.7) | 6,167 (32.4) |
| ≥82 | 6,461 (36.3) | 6,574 (35.6) | 7,385 (38.9) |
| Male sex, n (%) | 10,289 (57.8) | 10,539 (57.1) | 10,269 (54.0) |
| Body mass index on pretreatment (kg/m2), n (%) | |||
| <18.5 (underweight) | 1,795 (10.1) | 1,916 (10.4) | 2,150 (11.3) |
| 18.5–22.99 (low-normal weight) | 7,202 (40.4) | 7,376 (39.9) | 7,619 (40.1) |
| 23–24.99 (high-normal weight) | 3,306 (18.6) | 3,340 (18.1) | 3,257 (17.1) |
| 25–29.99 (overweight) | 3,227 (18.1) | 3,366 (18.2) | 3,218 (16.9) |
| ≥30 (obese) | 532 (3.0) | 556 (3.0) | 573 (3.0) |
| Missing | 1,748 (9.8) | 1,919 (10.4) | 2,189 (11.5) |
| Hypertension, n (%) | 8,995 (50.5) | 9,437 (51.1) | 9,190 (48.4) |
| Heart failure, n (%) | 2,576 (14.5) | 2,672 (14.5) | 3,228 (17.0) |
| Diabetes mellitus, n (%) | 3,465 (19.5) | 3,757 (20.3) | 4,059 (21.4) |
| Chronic obstructive pulmonary disease, n (%) | 82 (0.5) | 89 (0.5) | 101 (0.5) |
| Chronic kidney disease, n (%) | 334 (1.9) | 348 (1.9) | 353 (1.9) |
| Previous ischemic stroke, n (%) | 799 (4.5) | 845 (4.6) | 1,010 (5.3) |
| CHADs2 score on pretreatment, n (%) | |||
| 2 | 2,500 (14.0) | 2,569 (13.9) | 2,418 (12.7) |
| 3 | 6,807 (38.2) | 7,008 (37.9) | 7,108 (37.4) |
| 4 | 6,334 (35.6) | 6,571 (35.6) | 6,900 (36.3) |
| 5 | 2,010 (11.3) | 2,146 (11.6) | 2,365 (12.4) |
| 6 | 159 (0.9) | 179 (1.0) | 215 (1.1) |
| Ambulance use, n (%) | 11,034 (62.0) | 11,241 (60.9) | 12,025 (63.3) |
| Missing | 28 (0.2) | 12 (0.1) | 33 (0.2) |
| Unplanned admission or urgent admission, n (%) | 16,748 (94.0) | 17,221 (93.2) | 17,067 (89.8) |
| Missing | 28 (0.2) | 15 (0.1) | 33 (0.2) |
| Japan Coma Scale at admission, n (%) | |||
| Alert | 6,960 (39.1) | 7,182 (38.9) | 6,232 (32.8) |
| Dizziness | 8,086 (45.4) | 8,045 (43.6) | 8,187 (43.1) |
| Somnolence | 2,087 (11.7) | 2,460 (13.3) | 3,271 (17.2) |
| Coma | 677 (3.8) | 786 (4.3) | 1,316 (6.9) |
| Modified Rankin scale at admission, n (%) | |||
| 0 | 8,255 (46.4) | 8,270 (44.8) | 7,610 (40.0) |
| 1 | 2,448 (13.7) | 2,492 (13.5) | 2,238 (11.8) |
| 2 | 1,787 (10.0) | 1,914 (10.4) | 1,696 (8.9) |
| 3 | 1,504 (8.4) | 1,652 (8.9) | 1,601 (8.4) |
| 4 | 1,845 (10.4) | 1,850 (10.0) | 2,209 (11.6) |
| 5 | 1,044 (5.9) | 1,277 (6.9) | 1,946 (10.2) |
| Missing | 927 (5.2) | 1,018 (5.5) | 1,706 (9.0) |
| Computed tomography on pretreatment, n (%) | 15,871 (89.1) | 16,242 (87.9) | 15,155 (79.7) |
| Magnetic resonance imaging on pretreatment, n (%) | 14,705 (82.6) | 14,758 (79.9) | 12,993 (68.4) |
| Antiplatelet agents on pretreatment, n (%) | 2,943 (16.5) | 3,566 (19.3) | 2,924 (15.4) |
| Aspirin | 1,766 (9.9) | 2,149 (11.6) | 1,737 (9.1) |
| Ticlopidine | 14 (0.1) | 13 (0.1) | 15 (0.1) |
| Clopidogrel | 1,112 (6.2) | 1,433 (7.8) | 1,172 (6.2) |
| Prasugrel | 9 (0.1) | 4 (0.0) | 12 (0.1) |
| Cilostazol | 705 (4.0) | 877 (4.7) | 805 (4.2) |
| Sarpogrelate | 15 (0.1) | 10 (0.1) | 13 (0.1) |
| Antacid agents on pretreatment, n (%) | 9,584 (53.8) | 10,140 (54.9) | 9,896 (52.1) |
| Histamine-2 receptor antagonist | 5,068 (28.5) | 5,496 (29.8) | 5,593 (29.4) |
| Proton pump inhibitor | 5,154 (28.9) | 5,309 (28.7) | 5,019 (26.4) |
| Vonoprazan | 37 (0.2) | 50 (0.3) | 29 (0.2) |
| Hemodialysis or continuous renal replacement therapy on pretreatment, n (%) | 3 (0.0) | 3 (0.0) | 10 (0.1) |
| Mechanical ventilation on pretreatment, n (%) | 156 (0.9) | 183 (1.0) | 388 (2.0) |
| Edaravone on pretreatment, n (%) | 12,610 (70.8) | 12,720 (68.9) | 11,563 (60.8) |
| Glycerol on pretreatment, n (%) | 1,961 (11.0) | 2,771 (15.0) | 3,855 (20.3) |
| Ozagrel on pretreatment, n (%) | 1,353 (7.6) | 1,752 (9.5) | 1,807 (9.5) |
| Unfractionated heparin on pretreatment, n (%) | 11,154 (62.6) | 9,745 (52.8) | 7,676 (40.4) |
| Low-molecular-weight heparin on pretreatment, n (%) | 23 (0.1) | 32 (0.2) | 35 (0.2) |
| Warfarin on pretreatment, n (%) | 594 (3.3) | 659 (3.6) | 786 (4.1) |
| Endovascular procedures on pretreatment for stoke, n (%) | 948 (5.3) | 803 (4.3) | 761 (4.0) |
| Alteplase on pretreatment, n (%) | 2,219 (12.5) | 2,092 (11.3) | 1,885 (9.9) |
| Argatroban on pretreatment, n (%) | 2,296 (12.9) | 3,161 (17.1) | 2,451 (12.9) |
| Carbazochrome on pretreatment, n (%) | 148 (0.8) | 211 (1.1) | 503 (2.6) |
| Tranexamic acid on pretreatment, n (%) | 104 (0.6) | 152 (0.8) | 340 (1.8) |
| All reversal agents on pretreatment, n (%) | 139 (0.8) | 153 (0.8) | 277 (1.5) |
| Fresh frozen plasma | 16 (0.1) | 13 (0.1) | 50 (0.3) |
| Protamine | 84 (0.5) | 81 (0.4) | 102 (0.5) |
| Vitamin K | 48 (0.3) | 66 (0.4) | 154 (0.8) |
| Length of hospital stay, days [IQR] | 19 [12, 34] | 23 [15, 38] | 41 [26, 63] |
| Stroke care unit admission, n (%) | 2,374 (13.3) | 2,080 (11.3) | 1,517 (8.0) |
| Length of stay in stroke care unit, days [IQR] | 6 [4, 10] | 7 [4, 10] | 8 [5, 11] |
| Intensive care unit admission, n (%) | 1,360 (7.6) | 1,305 (7.1) | 1,432 (7.5) |
| Length of stay in intensive care unit, days [IQR] | 1 [1, 4] | 2 [1, 4] | 3 [2, 7] |
| Rehabilitation by physical therapist, n (%) | 9,193 (51.6) | 9,518 (51.5) | 8,269 (43.5) |
NOAC, non-vitamin K antagonist oral anticoagulants.
Table 2 shows the incidence proportion of primary or secondary outcomes divided by hospital days after initiating NOAC. Among all eligible patients, the incidence proportion of primary outcome in the early group was significantly lower than that in the late group (7.7% vs 9.7%, p < 0.01). The early group also had a significantly lower incidence proportion of all secondary outcomes compared with the late group. The proportion of gastrointestinal bleeding and intracranial hemorrhage was 8.6% (713/8,251 events) and 5.4% (445/8,251 events), respectively. The proportion of patients with a mRS value of 3 to 5 was 49.0% (7,244 of 14,785 patients) in the early group, 52.7% (8,230 of 15,611 patients) in the delayed group and 66.5% (10,830 of 16,281 patients) in the late group.
| Hospital days of initiating NOAC | Early (3–5 days) (N = 17,810) | Delayed (6–10 days) (N = 18,473) | Late (≥11 days) (N = 19,006) |
|---|---|---|---|
| Primary outcome | |||
| Hemorrhagic event after NOAC initiation or NOAC discontinuation, n % (95% CI) | 1,384 7.7 (7.3–8.1) | 1,468 7.9 (7.5–8.3) | 1,858 9.7 (9.3–10.1) |
| Secondary outcomes | |||
| Any hemorrhagic event, n % (95% CI) | 1,987 11.5 (11.1–12) | 2,269 12.5 (12.1–13) | 3,995 21 (20.4–21.6) |
| In-hospital death, n % (95% CI) | 487 2.8 (2.6–3.1) | 534 2.9 (2.7–3.2) | 799 4.3 (4–4.5) |
| Any thrombotic event, n % (95% CI) | 226 1.3 (1.1–1.5) | 316 1.7 (1.5–1.9) | 495 2.5 (2.3–2.8) |
| Deterioration of modified Rankin scale, n % (95% CI) | 9,451 53.1 (52.3–53.8) | 9,959 53.9 (53.1–54.6) | 10,561 55.6 (54.9–56.2) |
NOAC, non-vitamin K antagonist oral anticoagulants; CI, confidence interval.
Table 3 shows the results of multivariable logistic regression analyses for each outcome with adjustment with inverse probability of treatment weighting. Results from multivariable analysis for primary outcome found no significant association between the early group and the late group (adjusted odds ratio, 1.08; 95% confidence interval, 1.0–1.16). Otherwise, odds ratios for secondary outcomes in the late group were significantly higher than the early group.
| Early (3–5 days) | Delayed (6–10 days) | Late (≥11 days) | |
|---|---|---|---|
| Hemorrhagic event after initiating NOAC or discontinuation of NOAC | Reference | 0.98 (0.90–1.06) | 1.08 (1.00–1.17) |
| Any hemorrhagic event | Reference | 1.06 (1.00–1.14) | 1.73 (1.63–1.84) |
| In-hospital death | Reference | 1.00 (0.88–1.13) | 1.20 (1.06–1.35) |
| Any thrombotic event | Reference | 1.21 (1.01–1.44) | 1.88 (1.60–2.22) |
| Deterioration of modified Rankin scale | Reference | 1.06 (1.02–1.11) | 1.27 (1.22–1.33) |
NOAC, non-vitamin K antagonist oral anticoagulants.
Table 4 shows the results of multivariable logistic regression analyses as sensitivity analysis for primary or secondary outcomes. All the outcomes were associated with higher incidence proportion in the late group than the early group.
| 0–2 days | Early (3–5 days) | Delayed (6–10 days) | Late (≥11 days) | |
|---|---|---|---|---|
| Hemorrhagic event after initiating NOAC* or discontinuation of NOAC | 1.00 (0.93–1.08) | Reference | 1.00 (0.93–1.08) | 2.64 (2.47–2.82) |
| Any hemorrhagic event† | 1.08 (1.02–1.16) | Reference | 1.08 (1.02–1.16) | 2.89 (2.73–3.05) |
| In-hospital death | 1.00 (0.89–1.13) | Reference | 1.00 (0.89–1.13) | 1.33 (1.19–1.49) |
| Any thrombotic event | 1.26 (1.07–1.49) | Reference | 1.26 (1.07–1.49) | 1.76 (1.50–2.06) |
| Deterioration of modified Rankin scale | 1.12 (1.06–1.18) | Reference | 1.12 (1.06–1.18) | 1.51 (1.43–1.59) |
NOAC, non-vitamin K antagonist oral anticoagulants. *Hemorrhagic event after initiating NOAC excluded hemorrhagic event before NOAC initiation, †Any hemorrhagic event included all events either before or after NOAC initiation.
The proportions of the primary outcome in the additional sensitivity analysis are shown in Supplemental Table 1. The adjusted odds ratio for the primary outcome in the additional sensitivity analysis was 1.00 (95% confidence interval: 0.92–2.16) in the delayed group, and 1.00 (95% confidence interval 0.92–1.09) in the late group. The results of the subgroup analyses showed significant differences between NOAC initiation at 3 to 10 hospital days and ≥11 days in each group other than for JCS at admission and mechanical ventilation in pretreatment with respect to primary outcome (Supplemental Figure 1).
In the present study, we conducted a retrospective observational study using a nationwide Japanese database. The present study demonstrated that early NOAC initiation was not associated with subsequent hemorrhagic events or NOAC discontinuation among patients with AF-related AIS.
Several previous studies reporting the association between timing of the initiation of NOAC and outcomes were inconclusive. The Early Recurrence and Cerebral Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation (RAF) prospective cohort study reported data of 4–14 days after the initiation of anticoagulants from the onset of AF-related stroke [15]. In that study, 12.1% of the entire cohort were NOAC users. The results of the RELAXED study were inconsistent with those of the RAF study. They showed that initiation of rivaroxaban within 14 days after onset of ischemic stroke or transient ischemic attack was associated with a higher risk of major bleeding, although the lower recurrence of ischemic stroke was associated with the early (≤14 days) initiation of rivaroxaban [16]. Another analysis of a non-randomized cohort, the Virtual International Stroke Trials Archives (VISTA) [17], demonstrated that the early initiation of anticoagulants at 2–3 days after onset was associated with a favorable result for the recurrence of stroke and without excess risk of symptomatic intracerebral hemorrhage. The validity of the available expert consensus, the “1–3–6–12 day rule”, remains unclear to date. Although the clinical decision of when to initiate NOAC may depend on stroke severity, the present study found that NOAC initiation during the 3 to 5 days from admission may be preferable for preventing hemorrhagic events among patients with AF related AIS. Our results may serve to establish the best strategy for NOAC treatment for patients with AIS.
The present study showed no significant difference in the primary outcome between the delayed and early groups, although the delayed group was more likely to have any hemorrhagic event, any thrombotic event and deterioration of mRS compared with the early group. This result indicates that the delayed group may include patients with mild stroke who had a higher risk of bleeding. The proportion of prescription of aspirin, clopidogrel, or cilostazol in the delayed group was higher than that in the early group, but other background characteristics were not different, as shown in Table 1. Taking antiplatelet therapy may have affected the higher odds ratio in the delayed group.
Combining the results of the primary analysis with those of the additional sensitivity analysis indicated that initiating NOAC 11 or more days after AF-related AIS onset may not benefit a reduction in hemorrhage events. A possible reason for the additional analysis not indicating a significant difference may be explained by the possibility of deterioration of renal function in the primary analysis. Administration of NOAC to patients with estimated creatinine clearance under 30 mL/min for any NOAC other than apixaban or 15 mL/min for apixaban is not indicated in Japan. We cannot identify deterioration of chronic kidney disease in the database because laboratory test results are unavailable. However, any change in hemodynamic state by atrial fibrillation or mitral valve heart disease of differing severity may affect renal function in the course of a hospital stay.
In the present study, the secondary outcome analysis showed in-hospital death was significantly associated with the late timing of initiation of NOAC. It is persuasive that the increased occurrence of thrombotic events in the late initiation group may have provoked a higher mortality rate compared with the early group. Furthermore, there was a comparable increase in hemorrhagic events in the late group after NOAC initiation. Another possible reason for the increase in mortality may be the effects of increased thrombotic events combined with the increased incidence proportion of hemorrhagic events after NOAC. Indeed, the reported 30-day mortality or in-hospital death rate after anticoagulation for AF was associated with higher rates of intracranial hemorrhage than extracranial hemorrhage [18]. Gastrointestinal hemorrhage as a major hemorrhagic event in the present study was more prone to be treatable than intracranial hemorrhage. For the prevention of death after AIS, reducing the incidence proportion of intracranial hemorrhage can be a key strategy with careful decisions on timing of initiation of NOAC.
Prevalent use (NOAC prescription before ischemic stroke) of NOAC may be confounding between the timing of NOAC initiation and the primary outcome because NOAC prescription before ischemic stroke may affect the timing of NOAC initiation, recurrence of stroke, or hemorrhagic event. However, prescription information before index admission was not available in the database. Possible confounding may have been avoided by excluding patients who were prescribed NOAC within 2 days of admission. Although we acknowledge the discrepancy of the odds ratio for the late group between the main analyses and the sensitivity analyses on the primary outcome and any hemorrhagic event, the direction of effectiveness of exposure may be consistent between both analyses. The possible reason for the higher odds ratio in the sensitivity analyses may be an insufficient adjustment for covariates because we conducted multivariable analyses without a generalized boosted model as the sensitivity analysis.
The RAF study showed hazard ratios of hemorrhagic outcome of each NOAC initiation time compared with patients treated after 14 days [15]. These findings showed an increased direction of hazard ratio after 11 days. We consider that patients with NAOC administration after 11 days may have a higher risk for hemorrhagic events.
Although several studies reported that the early initiation of NOAC might provide benefit after AF-related AIS, making a decision on when NOAC should be initiated depends on the clinical practice because of limited evidence. Indeed, analyses of a Japanese prospective registry found that patients with a high score on the National Institutes of Health Stroke Scale were more likely to have a wider range (1–13 days after onset of index stroke or transient ischemic attack) of timing for the initiation of NOAC than patients with a low score by clinical practice [19]. Although impairment of conscious level at admission or delayed definite diagnosis may have delayed the timing of initiation of NOAC, the results from our analyses indicated that patients with late initiation of NOAC were more likely to have hemorrhagic events, thrombotic events or deaths. Sensitivity analysis showed the trend was similar to the primary analyses. The consistency of results of the present study suggested the robustness of the analyses.
An open-labeled randomized clinical trial, the Triple AXEL study [7], showed that rivaroxaban and warfarin administered within 5 days of stroke onset had identical results for safety and efficacy. Although the Triple AXEL study was a well-designed study, patients with a high risk of intracranial or systemic hemorrhage were excluded because it was an experimental study. Additionally, several randomized controlled trials investigating the efficacy of optimal timing of NOAC are upcoming (ELAN (NCT03148457; Switzerland), OPTIMAS (EudraCT, 2018-003859-38; UK), TIMING (NCT02961348; Sweden), and START (NCT03021928; USA)). The clinical effectiveness of the timing of the initiation of NOAC by AIS severity was investigated in the present study. We believe that our results add new insights for real-world practice.
There were several limitations in the present study. First, we conducted a retrospective study using a Japanese administrative inpatient database. No detailed data, including prescription information before admission, after discharge, size of infarction, hemorrhagic transformation, vital signs, and asymptomatic recurrence of stoke, were available because of the nature of the database. Nevertheless, an association between the timing of the initiation of NOAC and every outcome was robust after adjustment. Second, we included patients who tolerated the administration of NOAC. Thus, we performed analyses for all patients except those with the mildest and severest AF-related AIS, or a poor prognosis because of comorbidities. Third, the reason for NOAC prescription being stopped and whether patients actually took their NOAC prescription is not stated in the database. There may be misclassification of the primary outcome in the present study. Fourth, there may be potential overestimation of odds risk for any hemorrhagic event because of reverse causation.
Initiation of NOAC within 3–5 days was safer and associated with fewer thrombotic events, 90-day re-admission, and in-hospital death compared with after 11 days in patients with AF-related AIS.
This work was supported by the Ministry of Health, Labour and Welfare, Japan [grant numbers H29-Policy-Designated-009 and H29-ICT-General-004]; the Ministry of Education, Culture, Sports, Science and Technology, Japan [grant number 17H04141]; and the Japan Agency for Medical Research and Development (AMED).
None declared.
| Hospital days during which NOAC was initiated | Early (3–5 days) (N = 11,514) | Delayed (6–10 days) (N = 10,797) | Late (≥11 days) (N = 9,784) |
|---|---|---|---|
| Primary outcome | |||
| Hemorrhagic event after NOAC initiation or NOAC discontinuation, n (%) | 715 (6.2%) | 716 (6.6%) | 878 (9.0%) |
AF, atrial fibrillation; NOAC, non-vitamin K antagonist oral anticoagulants.

NOAC, non-vitamin K antagonist oral anticoagulants.