2014 Volume 47 Issue 3 Pages 85-94
2014 Volume 47 Issue 3 Pages 85-94
ATP-binding cassette (ABC) transporters are involved in chemotherapy resistance. Multidrug-resistance protein 8 (ABCC11/MRP8) is also involved in 5-fluorouracil (5-FU) metabolism. 5-FU and its derivatives are widely used in the treatment of gastrointestinal tract cancers, but little is known about the contribution of ABCC11/MRP8 to gastrointestinal tract and related cancers. Here, we report our investigation of ABCC11/MRP8 expression in normal and cancerous gastrointestinal tract tissues and reveal its novel role in the gastric mucosa. In tissue microarray and surgically resected cancer specimens, immunohistochemical (IHC) staining revealed significantly reduced expression of ABCC11/MRP8 in gastrointestinal tract cancers compared with other cancers. In contrast, strong ABCC11/MRP8 expression was observed in normal gastric mucosa. Additional immunofluorescence assays revealed co-localization of ABCC11/MRP8 and pepsinogen I in normal gastric chief cells. Quantitative PCR and Western blot analysis also revealed significant expression of ABCC11/MRP8 in fundic mucosa where the chief cells are mainly located. Furthermore, the ABCC11 mRNA-suppressed NCI-N87 gastric cancer cell line failed to secret pepsinogen I extracellularly. Thus, low expression of ABCC11/MRP8 is consistent with chemotherapeutic regimens using 5-FU and its derivatives in gastrointestinal tract cancers. Our results indicated a novel function of ABCC11/MRP8 in the regulation of pepsinogen I secretion in the normal gastric chief cells.
The ATP-binding cassette (ABC) genes represent the largest family of transmembrane proteins. These proteins bind ATP and use the energy to drive the transport of various molecules across all cell membranes [6]. Until today, 48 ABC transporters have been identified in humans [8, 22, 28]. Among those, ABC transporters, sub-family C, member 11, coding multidrug-resistance protein 8 (ABCC11/MRP8) was first cloned from a cDNA library derived from normal human breast and liver [8, 22, 28]. It contains two conserved nucleotide-binding domains and 12 putative transmembrane domains classified as “full transporters” [22].
ABC transporters confer drug resistance against a broad range of chemotherapeutic agents [8]. ABCC11/MRP8 has been shown to act as a metabolic apparatus of 5‑fluorouracil (5-FU) [14], which is widely used in cancer chemotherapy [16]. 5-FU and its derivatives are metabolized to 5-fluoro-2'-deoxyuridine 5'-monophosphate (FdUMP) by intracellular metabolic enzymes [16, 24, 25]. FdUMP is actively transported by ABCC11/MRP8, which has been suggested to be involved in conferring resistance to 5-FU [4, 5, 7, 31, 36]. In gastrointestinal tract cancers, 5-FU-based drugs are ordinarily selected for first-line chemotherapy, and these drugs are known to improve both overall and disease-free survival [25, 32].
On the other hand, ABCC11/MRP8 has specific roles in secretory organs. Our previous studies demonstrated that ABCC11/MRP8 was responsible for earwax secretion [27, 35]. In summary, a SNP (538G>A, Gly180Arg) in the ABCC11 gene determines the type of earwax, and the GG homozygous and GA heterozygous genotypes correspond to wet earwax [35]. Similarly, ABCC11 wild type is responsible for the secretion of pre-odoriferous compounds from the axillary apocrine gland [27], and associated with axillary osmidrosis [11, 21, 30]. In addition, ABCC11/MRP8 plays a role in colostrum secretion by acting as an efflux pump and is a peripheral factor independent of endocrine control [20]. These findings and the reported variety of transport molecules associated with ABCC11/MRP8 suggest that it may also have some effect on other secretory organs.
ABCC11/MRP8 is expressed in normal tissues including the brain, breasts, lungs, liver, kidney, placenta, prostate, testes, and apocrine glands, as well as in cancerous tissues [3, 4, 34]. However, the contribution of ABCC11/MRP8 to gastrointestinal tract and related cancers remains poorly understood. The main purpose of this study was to clarify the expression profile of ABCC11/MRP8 and its possible function in gastrointestinal tract and related cancers.
Tissue samples were obtained with informed consent from 87 patients treated surgically at Nagasaki University Hospital (Table 1). Formalin-fixed paraffin-embedded (FFPE) sections were subjected to immunohistochemical (IHC) staining and immunofluorescence (IF) assay. Fresh frozen samples of normal gastric mucosa were also obtained from gastric cancer patients. A tissue microarray (Human Multiple Normal and Cancer Tissue Array, Protein Biotechnologies, Ramona, CA, USA) containing 48 normal tissues and 48 solid cancers was also used to analyze ABCC11/MRP8 expression.
| n (male, female) | Mean Age (range) | IHC Staining Score | |
|---|---|---|---|
| Breast Cancer | 14 (0, 14) | 61.9 (49–88) | 1.75±0.51*** |
| HCC/CCC | 10 (10, 0) | 67.2 (53–76) | 1.65±0.88** |
| Pancreatic Cancer | 5 (2, 3) | 67.6 (52–81) | 1.00±0.35* |
| Lung Cancer | 19 (14, 5) | 65.1 (42–79) | 1.05±0.69** |
| Thyroid Cancer | 5 (2, 3) | 51.4 (24–76) | 0.80±0.76 |
| Gastrointestinal tract Cancer | 34 (17, 17) | 64.1 (43–88) | 0.44±0.54 |
| Esophageal | 5 (3, 2) | 65.4 (63–69) | 0.70±0.57 |
| Gastric | 16 (6, 10) | 61.7 (37–79) | 0.25±0.45 |
| Colorectal | 13 (8, 5) | 66.7 (43–82) | 0.54±0.59 |
| Total | 87 (45, 42) | 63.8 (24–88) | 1.00±0.80 |
n, number of patients analyzed; HCC, hepatocellular carcinoma; CCC, cholangiocellular carcinoma.
IHC Staining score was calculated by two investigators using clarification criteria as described. Data represent mean values±SD.
*/ **/ ***; significant difference between gastrointestinal tract cancer and cancers of other organs, P<0.05 (*), P<0.01 (**), and P<0.001 (***), based on a nonparametric Mann–Whitney U test.
Human gastric cancer cell line NCI-N87 (CRL 5822) was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Human gastric cancer cell line HGC-27 (RCB 0500), which did not have the morphological and enzyme-histochemical characteristics of glandular epithelial cells [1], was provided by RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan. These cell lines were characterized and authenticated at the repository by methods such as mycoplasma infection testing and DNA identification testing (short tandem repeat analysis) and were used within 6 months of purchase. The cells were maintained in RPMI 1640 (Life Technologies, Carlsbad, CA, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Life Technologies) and 1% (v/w) penicillin/streptomycin (Life Technologies). Cells were subcultured using 0.05% trypsin-0.53 mM EDTA solution (Life Technologies). For the ABCC11 mRNA suppression assay, cells were cultured in Opti-MEM I serum-free medium (Life Technologies).
AntibodiesFor IHC staining, primary antibodies were used at the following concentrations: rabbit polyclonal anti-human ABCC11/MRP8 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), 1:200 and mouse monoclonal anti‑human pepsinogen I antibody (Sanbio BV, Uden, The Netherlands), 1:500. Horseradish peroxidase (HRP)-conjugated goat polyclonal anti-rabbit IgG (Nichirei Biosciences, Tokyo, Japan) and HRP-conjugated goat poly‐clonal anti-mouse IgG (Nichirei Biosciences) were used as detecting antibodies. Alexa Fluor 488-conjugated goat polyclonal anti-rabbit IgG (Life Technologies), 1:2000 and Alexa Fluor 594-conjugated goat polyclonal anti-mouse IgG (Life Technologies), 1:2000 were also used as detecting antibodies in the IF assay. In the Western blot analysis, additional mouse monoclonal anti-actin antibody (Abcam, Cambridge, MA, USA) or anti-GAPDH antibody were used as internal controls.
Immunohistochemical staining and immunofluorescence assayFFPE sections were deparaffinized in dimethylbenzene and rehydrated through a graded alcohol series. After antigen retrieval in HistoVT One (Nacalai Tesque, Kyoto, Japan) (95°C, 20 min), blocking of endogenous peroxidase activity in 3% H2O2 solution, sections were incubated with each primary antibody (4°C, overnight). After washing in PBS, sections were incubated with the detecting antibody (room temperature, one hour). For IHC staining, sections were visualized with 3,3'-diaminobenzidine tetra hydrochloride (DAB: brown) or 3-amino-9-ethylcarbazole (AEC: red) and counterstained with hematoxylin. The sections visualized with DAB were dehydrated with alcohol and dimethylbenzene and mounted in a conventional fashion. Sections visualized with AEC were mounted in aqueous media without dehydration. Normal breast tissue specimens, which moderately expressed ABCC11/MRP8 [4] were prepared as positive controls in all cases. Negative controls were also prepared in all cases by substituting the primary antibody. The IHC staining scores were calculated by two investigators using the following criteria: score 0, no expression; score 1, low expression <10%; score 2, moderate expression >10% or diffuse staining; score 3, strong expression >90%, or strong focal staining. The mean values were determined for each cancer.
For IF assay, sections were mounted in mounting media with 4',6-diamidino-2-phenylindole (DAPI). Double IF assays were observed by confocal laser scanning microscopy (LSM 510 META, Carl Zeiss, Oberkochen, Germany) or fluorescence microscopy (BX70, Olympus, Tokyo, Japan).
ELISAThe amount of pepsinogen I secreted in culture medium was measured by specific ELISA tests (Biohit Plc, Helsinki, Finland). All experiments were performed in duplicate. The mean values of each sample were normalized by the concentration of the total protein.
Western blot analysisTo extract whole proteins, the gastric mucosa specimens were homogenized in radio-immunoprecipitation assay (RIPA) buffer (Nacalai Tesque) using a Bioruptor (Tosho Denki, Yokohama, Japan). Following centrifugation at 5,000 g for 10 min, the supernatant fraction was collected. For culture samples, the supernatant fraction of each culture medium was collected. Whole cell lysates were prepared as cytoplasmic fractions using RIPA buffer (Nacalai Tesque). The protein concentrations of all samples were quantified using a BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The same amount of protein from each sample was prepared in a sample buffer (ATTO, Tokyo, Japan) containing SDS. The proteins were then separated by 8% SDS-PAGE (Tefco, Tokyo, Japan) and electro-transferred to polyvinylidene difluoride (PVDF) membranes (GE Healthcare, Buckinghamshire, UK). One hour after blocking in Tris-buffered saline containing 0.1% (v/v) Tween 20 (TBS-T) and 1% (w/v) skim milk, primary and detecting antibodies were used at their own concentrations. HRP-dependent luminescence was developed using ECL Plus Western Blotting Detecting Reagents (GE Healthcare), and was detected with a Lumino Imaging Analyzer FluorChem Imaging System (Cell Biosciences, Santa Clara, CA, USA) according to the manufacturer’s specifications.
Total RNA extraction and quantitative PCRTotal RNA was extracted using High Pure RNA Isolation Kit (Roche Diagnostics, Mannheim, Germany). Reverse transcription was performed with 2 μg of the total RNA using High-Capacity Reverse Transcription (Life Technologies). Quantitative PCR analysis was performed using a Life Technologies Prism 7900HT Sequence Detection System. TaqMan probes and primers for ABCC11/MRP8 were obtained as assay-on-demand gene expression products (assay ID: Hs01090768_m1, Life Technologies). The mRNA of pepsinogen I was analyzed using the following primer set (sense primer, 5'-CCC GTC TTT GAC AAC ATC TG-3'; anti-sense primer, 5'-CGC TGC CAC TCT TGT CAT C-3'). The mRNA of GAPDH was used as an endogenous control (assay ID: Hs99999905_m1, Life Technologies). The thermal cycler conditions were as follows: held for 10 min at 95°C followed by two-step PCR for 45 cycles of 95°C for 15 sec and 60°C for 1 min. All experiments were performed in quadruplicate. The number of transcripts was calculated from a standard curve obtained by plotting the known input of six different concentrations versus the PCR cycle number at which the detected fluorescence intensity reached a fixed value. Amplification data were analyzed with Prism Sequence Detection Software version 2.1 (Life Technologies). For each sample, data were normalized to GAPDH.
ABCC11 mRNA suppression by RNA interferenceCells were suspended in serum-free Opti-MEM I medium (Life Technologies) at a concentration of 105 cells/well. Small interfering RNA (siRNA) for ABCC11 (predesigned siRNA, ID: s39907) and negative-control siRNA (Silencer negative control siRNA, ID: AM4611) were purchased from Life Technologies. Prepared cells were transfected with siRNA according to the manufacturer’s specifications using 5 μl siPORT NeoFX Transfection agent (Life Technologies) to produce a final RNA concentration of 50 nmol/l in serum-free medium. The cells were harvested after two days of transfection. The efficiency of siRNA transfection was determined by quantitative PCR, and WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt] [12] (Nacalai Tesque) assay was also performed to determine cell viability.
Statistical analysisStatistical significance was assessed using either unpaired Student’s t-test for parametric data or Mann–Whitney U test for nonparametric data. StatView software version 5.0 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Representative positive- and negative-stained cancer specimens obtained from cancer patients are shown in Figure 1. Genetically, the expression of ABCC11/MRP8 was proved in normal tissues of liver and breast by PCR [3, 34]. In our experiments, almost all specimens of HCC and breast cancer were ABCC11/MRP8 positive, and they were observed in the cytoplasm (Fig. 1a and b). In contrast, almost all specimens of gastric cancer and colon cancer were negative (Fig. 1c and d).

Representative positive- and negative-stained cancer specimens obtained from cancer patients. a, HCC (moderately differentiated); b, breast cancer (invasive ductal carcinoma); c, gastric cancer (moderately differentiated adenocarcinoma); d, colon cancer (well differentiated adenocarcinoma). Upper specimens are ABCC11/MRP8 positive and lower specimens are negative.
Representative positive- and negative-stained cancer specimens in the tissue microarray (48 normal tissues and 48 cancers) are shown in Figure 2A. ABCC11/MRP8 expression in 87 cancer patients was analyzed using the IHC Staining Score based on the classification criteria. In gastrointestinal tract cancers, ABCC11/MRP8 expression was significantly lower than that in breast, HCC/CCC, pancreatic, and lung cancers (Fig. 2B).

A: Representative ABCC11/MRP8 staining of the tissue microarray. a and b, lung cancer (adenocarcinoma); c and d, breast cancer (infiltrating ductal carcinoma); e and f, colon cancer (mucinous adenocarcinoma); g, gastric cancer (mucinous adenocarcinoma). Upper specimens are ABCC11/MRP8 positive and lower specimens are ABCC11/MRP8 negative. Note that all gastric cancers are ABCC11/MRP8 negative. Bar=200 μm. B: ABCC11/MRP8 IHC Staining Scores for 87 surgically resected sections. HCC, hepatocellular carcinoma; and CCC, cholangiocellular carcinoma. Dot plots are representative of each IHC Staining Score, and horizontal bars represent the mean values. Statistical significance between cancer types was determined by a nonparametric Mann–Whitney U test. *, P<0.05; **, P<0.01; and ***, P<0.001.
ABCC11/MRP8 expression was observed in the normal gastric mucosa. In low magnification images, ABCC11/MRP8 immunopositive cells are gathered in the fundic glands similarly to pepsinogen I (Fig. 3A). In high magnification images, positive cells contained vacuolar structures in the cytoplasm, and the nuclei were pushed aside, suggesting that these were gastric chief cells (Fig. 3B). In contrast, larger, round gastric parietal cells were not stained with ABCC11/MRP8 and pepsinogen I.

A: Representative ABCC11/MRP8 and pepsinogen I staining images in serial sections of normal gastric mucosa obtained from three different patients. B: ABCC11/MRP8 and pepsinogen I staining in serial sections of surgically resected normal gastric mucosa at high magnification. Black arrowheads indicate gastric chief cells, and white arrowheads indicate gastric parietal cells.
To clarify the co-localization of ABCC11/MRP8 with pepsinogen I, double immunofluorescent (IF) assay was performed. In double IF assay of the normal gastric mucosa, ABCC11/MRP8-and pepsinogen I-expressing cells were completely matched (Fig. 4A and B). Pepsin-producing NCI-N87 cells [2, 20] were also immunopositive for ABCC11/MRP8 and pepsinogen I, co-localizing in the cytoplasm (Fig. 4C).
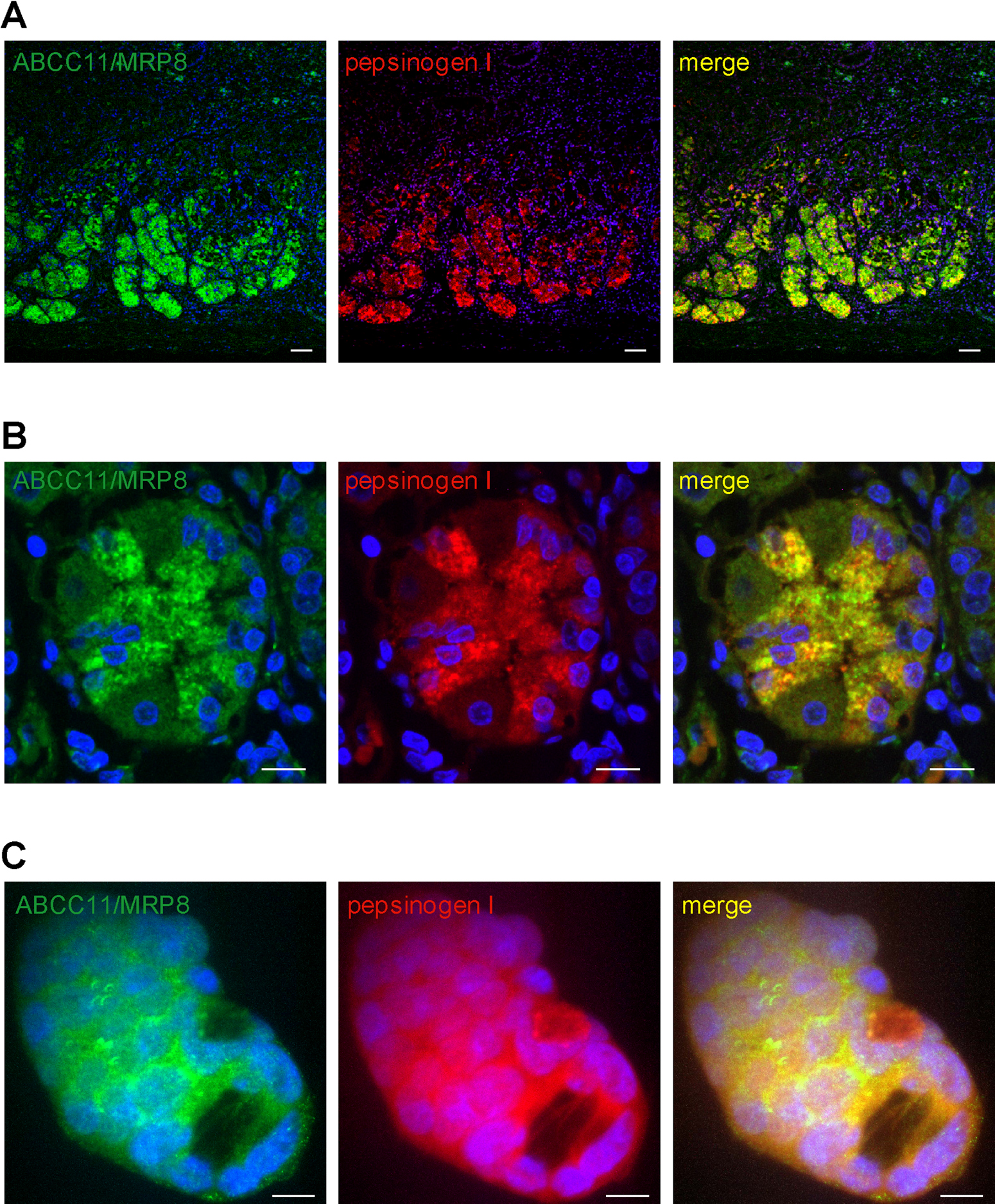
Double immunofluorescence assay of surgically resected normal gastric mucosa and NCI-N87 cells with the following fluorescence-labeled antibodies: green, ABCC11/MRP8; red, pepsinogen I; and blue, DAPI. A: Low magnification of normal gastric mucosa, confocal laser scanning microscopy. Bar=50 μm. B: High magnification of normal gastric mucosa, confocal laser scanning microscopy. Bar=10 μm. C: High magnification of NCI-N87 cells fixed with 3.7% paraformaldehyde, fluorescence microscopy. Bar=10 μm.
Quantitative PCR assay revealed significant expression of ABCC11/MRP8 in gastric fundic mucosa (P<0.005), which overlapped the distribution of gastric fundic glands (Fig. 5A). Western blot analysis of ABCC11/MRP8 also showed limited localization in fundic mucosa (Fig. 5B).

Localization of ABCC11/MRP8 in the normal gastric mucosa was investigated using specimens from surgically resected patients. A: Quantitative real-time PCR analysis of 19 specimens for four parts of the normal gastric mucosa: upper, cardia, and fundus; middle, body of stomach; lower, pyloric antrum and pyloric canal; and duodenum, superior part of the duodenum. The right schematic shows the definitions of each part of the stomach. Data represent the mean±SD. Statistical significance was determined by unpaired Student’s t-test. **, P<0.01 and ***, P<0.001. B: Western blot analysis of gastric cancer cell lines (NCI-N87 and HGC-27) and normal gastric specimens from four parts of the gastric mucosa. GAPDH was used as the internal control protein.
NCI-N87 cells secret pepsin and they have been used as a model of human gastric epithelial functions [2, 20]. To investigate the contribution of pepsinogen I secretion by ABCC11 mRNA expression, NCI-N87 cells were used in an ABCC11 mRNA suppression assay with siRNA. Figure 6A shows almost 80% reduction in ABCC11 expression in NCI-N87 cells transfected with ABCC11 siRNA compared with control cells. ELISA assay showed that the ABCC11 reduced by siRNA almost abolished pepsinogen I secretion to the extracellular culture medium without decreasing cell viability (P<0.005 vs. negative siRNA) (Fig. 6B and C). Although the cells transfected with ABCC11 siRNA failed to secrete pepsinogen I extracellularly, cytoplasmic pepsinogen I mRNA and pepsinogen I were not altered (Fig. 6D and E).

Alteration of pepsinogen I secretion by ABCC11 siRNA was investigated using NCI-N87 cells. A: Quantitative real-time PCR analysis of ABCC11 mRNA two days after siRNA transfection. Data were normalized to housekeeping gene GAPDH mRNA. Graph shows the relative ABCC11 expression compared with negative siRNA as 100%. Data represent mean±SD. B: Secreted pepsinogen I in each culture medium measured by ELISA two days after siRNA transfection. Graph shows relative pepsinogen I secretion compared with negative siRNA as 100%. Statistical significance was determined by unpaired Student’s t-test. ***, P<0.001. C: Cell viability was evaluated by WST-8 assay to investigate the cytotoxicity associated with the transfection agents or manipulations at two days after transfection. Graph shows the relative viability compared with negative siRNA as 100%. Data represent the mean±SD. Statistical significance was determined by unpaired Student’s t-test. n.s., not significant. D: Quantitative real-time PCR analysis of pepsinogen 1 mRNA at two days after siRNA transfection. Data were normalized to housekeeping gene GAPDH. Graph shows the relative pepsinogen 1 mRNA expression compared with negative siRNA as 100%. Data represent the mean±SD. E: Western blot analysis for pepsinogen I. Cytoplasmic proteins from each fraction of NCI-N87 cells transfected with ABCC11 siRNA were extracted at two days after transfection in triplicate. NCI-N87 cells transfected with negative siRNA were used as a positive control, and HGC-27 cells were used as a negative control. Actin was used as the internal control protein.
In the present study, we investigated the expression of ABCC11/MRP8 in various cancers and revealed that this expression was reduced in gastrointestinal tract cancers compared with breast, HCC/CCC, pancreatic, and lung cancers. ABCC11/MRP8 has been reported to contribute to multidrug-resistance especially 5-FU and its derivatives [24, 31, 36]. Our finding of reduced ABCC11/MRP8 expression in gastrointestinal tract cancers supports the view that these cancers are sensitive to 5-FU and its derivatives, and this is consistent with findings from routine chemotherapy regimens [16]. The investigation of expression level of ABCC11/MRP8 may provide us with useful information for making choices when tailor-making chemotherapies as well as for evaluating histoculture drug response assay or collagen gel droplet embedded culture drug sensitivity test. Hlavata and colleagues analyzed the relations between transcript levels of all known ABC transporters in colorectal cancers and chemotherapy efficiency, and revealed that ABCC11/MRP8 might be a promising candidate marker for 5-FU therapy [10]. Furthermore, Uemura and colleagues demonstrated that ABCC11/MRP8 directly confers resistance to pemetrexed, a new-generation antifolate antimetabolite [31]. In gastrointestinal tract cancers, chemotherapies with pemetrexed are currently under investigation. Our findings might add further insight on the potential clinical benefits of pemetrexed in gastrointestinal tract cancers.
In the present study, the over-expressions of ABCC11/MRP8 were not observed in 16 gastric cancer patients. However, reported cell line based studies have demonstrated that increased expression of ABCC11/MRP8 directly conferred chemoresistance [24, 31]. Considering the colorectal cancer studies, the relations between the expression levels of ABCC11/MRP8 and chemoresistance to 5-FU might be observed also in gastric cancers. Additional investigations are required to elucidate the possible role of biological effect of ABCC11/MRP8 for chemoresistance in gastric cancers.
We also intensively analyzed ABCC11/MRP8 expression in normal gastric mucosa because of the strong immunohistochemical staining in gastric chief cells. To the best of our knowledge, the present study is the first to reveal the expression of ABCC11/MRP8 in normal gastric mucosa. Interestingly, ABCC11/MRP8 expression was observed in normal gastric mucosa. However, it was almost negative in gastric cancers regardless of their histological types or differentiations (poorly adenocarcinoma; 6, signet ring cell carcinoma; 5, moderately adenocarcinoma; 3, others; 3). This discrepancy may be explained by the origin of gastric cancers. In humans, intestinal metaplasia and spasmolytic polypeptide-expressing metaplasia (SPEM) are associated with the precancerous stomach [33]. Nozaki and colleagues reported that gastric chief cells transdifferentiated into SPEM cells with down-regulated mature chief cell markers including pepsinogen I [23].
Pepsinogen I is secreted by gastric chief cells in fundic glands around the upper stomach. Pepsinogen II is secreted in the pyloric and Brunner’s glands around the lower stomach as well as fundic gland [9, 13]. The present study indicated that ABCC11/MRP8 was expressed in the upper portion of the gastric mucosa. Therefore, we hypothesized that ABCC11/MRP8 regulated pepsinogen I secretion, and not pepsinogen II. Pepsinogen synthesis and secretion are regulated by positive and negative feedback mechanisms [26]. In theory, pepsinogens are synthesized on the endoplasmic reticulum and modified at the Golgi body, then, once stored in granules, they are secreted through an exocytosis process [9, 26]. The releasing process and modulators that bind the gastric chief cell receptors with specific antagonists have been studied intensively. However, the mechanism by which they are sequestered into granules and moved to the plasma membrane for exocytosis has not been identified. In the present study, ABCC11-knocked down cells could synthesize pepsinogen I intracellularly, but failed to secrete it extracellularly. Considering the molecular weight of the pepsinogen I, it may be too large for a transportation substrate of ABCC11/MRP8 [36]. Thus, the mechanism of reducing pepsinogen I with ABCC11-knockdown might be an indirect suppression, through some smaller molecule essential to the generating process of pepsinogen I. Although further research is necessary for elucidation of the mechanisms, our results suggest that ABCC11/MRP8 might play a crucial role in the preliminary steps of pepsinogen I exocytosis.
Previously, we reported the functional modification of ABCC11/MRP8 that determined the phenotype of earwax by a SNP of the ABCC11 gene [35]. Similarly, ABCC11 genotype is responsible for the secretion in the axillary apocrine gland [29], and axillary osmidrosis [11, 20, 30]. In addition, ABCC11 genotype is also responsible for secretion of colostrum [20]. Considering our experimental data and the studies cited here, ABCC11/MRP8 might be a key molecule of “exocrine” function in various tissues. It is still unknown whether the SNP genotype correlates with pepsinogen I secretion. Serum pepsinogen tests were introduced for mass screening of chronic gastritis and gastric cancer [15, 17–19]. Therefore, it would be clinically important to investigate the SNP genotype and pepsinogen I. Additional studies of ABCC11/MRP8 including SNP genotyping and secretion models might help to clarify the mechanism of exocrine systems in the gastrointestinal tract.
In conclusion, low expression of ABCC11/MRP8 was found in gastrointestinal tract cancers. Our findings would have some implications for the rational understanding of chemoresistance in gastrointestinal tract cancers. Furthermore, our findings also indicate a novel function of ABCC11/MRP8 in normal gastric chief cells, which regulate pepsinogen I secretion, possibly in the process of exocytosis. These results might give us a new insights into pepsinogen production and ABCC11/MRP8 regulation in gastric diseases.
The authors declare no potential conflicts of interest.
The authors would like to thank Prof. M. Oka (Department of Respiratory Medicine, Kawasaki Medical School), Prof. K. Tsukamoto (Department of Pharmacotherapeutics, Nagasaki University Graduate School of Biomedical Sciences), and Dr. N. Miwa (Department of Human Genetics, Nagasaki University Graduate School of Biomedical Sciences) for their support and advice during the study.
The study was not supported by any sponsors during the study design and data collection, analysis, and interpretation phases as well as during the writing of the report.