2014 Volume 47 Issue 3 Pages 95-102
2014 Volume 47 Issue 3 Pages 95-102
In the major salivary glands of mice, acinar cells in the parotid gland (PG) are known to be the main site for the production of the digestive enzyme α-amylase, whereas α-amylase production in the submandibular gland (SMG) and sublingual gland (SLG), as well as the cell types responsible for α-amylase production, has been less firmly established. To clarify this issue, we examined the expression and localization of both the mRNA and protein of α-amylase in the major salivary glands of male and female mice by quantitative and histochemical methods. α-amylase mRNA levels were higher in the order of PG, SMG, and SLG. No sexual difference was observed in α-amylase mRNA levels in the PG and SLG, whereas α-amylase mRNA levels in the female SMG were approximately 30% those in the male SMG. Using in situ hybridization and immunohistochemistry, signals for α-amylase mRNA and protein were found to be strongly positive in acinar cells of the PG, serous demilune cells of the SLG, and granular convoluted tubule (GCT) cells of the male SMG, weakly positive in seromucous acinar cells of the male and female SMG, and negative in mucous acinar cells of the SLG. These results clarified that α-amylase is produced mainly by GCT cells and partly by acinar cells in the SMG, whereas it is produced exclusively by serous demilune cells in the SLG of mice.
The major salivary glands of rodents, as well as those of humans, are composed of the parotid gland (PG), submandibular gland (SMG), and sublingual gland (SLG) [4, 20, 28]. The acinar systems of the PG and SLG of rodents contain serous acinar cells in the former and mucous acinar cells accompanied by serous demilune cells in the latter, similar to those of humans. In contrast, the acinar system of the SMG of rodents, unlike that of humans, contains a single type of seromucous acinar cells, and this produces both mucin glycoproteins, which is a characteristics of mucous cells, and various bioactive proteins as well as water and ions, which is a characteristics of serous cells. Furthermore, the duct system of the rodent SMG differs from that of the human SMG, in that a special duct portion called the granular duct or granular convoluted tubule (GCT) exists between the intercalated duct (ID) and striated duct (SD) [10]. During the postnatal growth of rodents, especially of mice, extensive development of the GCT from the SD takes place preferentially in males during puberty, resulting in a marked sexual difference in the morphology and function of the duct system in adults [2, 3]. The epithelial cells of the GCT have abundant secretory granules that contain various bioactive peptides such as nerve growth factor (NGF) and epidermal growth factor (EGF) [1, 6].
Amylase is a digestive enzyme for polysaccarides that is widely distributed in nature and classified into several types. α-amylase is the main type of amylase in mammals, including humans and rodents, with molecular weights 50–60 kDa, whereas β-amylase is produced by bacteria, fungi, and plants but not by animals [12]. α-amylase catalyzes the hydrolysis of α-1,4-glucan bonds in starch with the calcium dependence and the optimum pH of 6.7–7.0. This enzyme is further divided into two isozymes, i.e., salivary and pancreatic α-amylases, that are coded by different genes [8]. In rodents, it is generally accepted that the PG is the primary source of salivary α-amylase, while the contribution of the SMG and SLG is markedly smaller. The production of α-amylase by the human [16] and rat PG [22] was initially described in terms of the catalytic activity levels of parotid homogenates. Immunohistochemistry (IHC) demonstrated the localization of α-amylase in the secretory granules of acinar cells in the human [11], rat [26] and mouse PG [27]. Furthermore, in situ hybridization (ISH) supported the acinar cell localization of α-amylase transcripts in the human PG [15]. In contrast, the expression and cellular localization of α-amylase in the SMG and SLG has been less firmly established. In earlier studies, α-amylase activity in the homogenate of mouse SMG was found to increase preferentially in males after puberty in parallel with the development of GCT cells, resulting in sexually dimorphic levels in adult ages [7]. The histochemical starch substrate-film technique detected greater α-amylase activity in the areas of GCT than acini [24, 25]. While these results strongly suggest the GCT cell localization of α-amylase in the mouse SMG, immunohistochemical evidence for α-amylase localization has been limited and controversial. Earlier studies detected α-amylase immunoreactivity in the secretory granules of acinar cells only and not in those of GCT cells, in spite of the preferential presence of α-amylase enzyme activity in the latter [18, 27]. α-amylase immunoreactivity was later detected at the electron microscopic level in the secretory granules of GCT cells in the mouse SMG [13, 14]. Regarding the SLG, the substrate-film technique either failed to detect α-amylase activity in the mouse SLG [25], or localized it in the stroma around acini [27], whereas the immunoelectron microscopic localization of α-amylase was reported in the secretory granules of serous demilune cells in the mouse [13, 14] and gerbil SLG [9]. An analysis of the expression and localization of α-amylase mRNA has not yet been conducted in the SMG or SLG of mice, which would provide powerful evidence for the production of α-amylase protein.
Therefore, we aimed to re-evaluate the expression and localization of salivary α-amylase in the SMG and SLG of mice using quantitative RT-PCR and ISH in combination with IHC.
Male and female C57BL6 mice at the age of 8 weeks (W) were purchased from Nippon SLC (Hamamatsu, Japan), reared under standard 12 hr light/12 hr dark laboratory conditions with free access to standard food and water, and used at 9–10 W old (adult). All subsequent experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals at Kanazawa University. Male and female animals under various experimental conditions were sacrificed under pentobarbital anesthesia by transcardial perfusion with physiological saline. The SMG, SLG, and PG were removed, frozen immediately in liquid nitrogen, and stored at –80°C until use for RT-PCR and Western blot analyses. Animals were fixed by perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.2, and the salivary glands were removed and further fixed by immersion in the same fixative overnight at 4°C for ISH and IHC analyses. Specimens were then either rinsed overnight at 4°C with 30% sucrose in 0.1 M phosphate buffer, frozen, and cut into 8 μm sections using a cryostat for ISH, or dehydrated in a graded ethanol series, embedded in paraffin, and cut into 4 μm sections using a microtome for IHC. Cryostat and paraffin sections were then mounted on silanized glass slides (DAKO, Glostrup, Denmark).
RNA preparation and RT-PCRTotal RNA was isolated from the frozen specimens using a miRNAeasy Mini Kit (Quiagen, Venlo, Netherlands). First-strand cDNA was synthesized from 2 μg aliquots of total RNA samples using the oligo(dT)20 primer and Moloney murine leukemia virus reverse transcriptase (Toyobo, Osaka, Japan). cDNA fragments were amplified from these RT products with conventional and quantitative RT-PCR. The sequences of the primer pairs used in the present study are as follows: α-amylase primers (forward: actgggctttgtcagaaact, reverse: gggtcttcggcagagttact); NGF primers (forward: tcgactccaaacactggaac, reverse: gtcagcctcttcttgtagcc); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers (forward: accacagtccatgccatcac; reverse: tccaccaccctgttgctgta). Conventional RT-PCR was first performed for 25–30 cycles using TaqDNA polymerase (ExTaq; Takara Biomedicals, Kusatsu, Japan) in a PCR Thermal Cycler Dice (Takara), and the amplified cDNA fragments were analysed with agarose-gel electrophoresis.
Real-time quantitative RT-PCR for α-amylase transcripts was performed in a Stratagene Mx-3005P Thermocycler (Stratagene, La Jolla, CA) according to the procedures recommended by the manufacturer. Each reaction mixture contained Brilliant II SYBR Green QPCR Master Mix (Agilent Technologies, Santa Clara, CA), RT products, and primer pairs for the gene of interest. The transcripts for GAPDH were also amplified as an internal control to normalize the data. The reaction was performed for 10 min at 95°C followed by 45 cycles at 95°C for 40 sec, 60°C for 30 sec, and 72°C for 30 sec, and the relative levels of transcripts in total RNA samples were evaluated with the ΔΔCt method using MxPro QPCR software (Agilent Technologies, Santa Clara, CA). Each sample was analyzed in triplicates and samples from 4 or 5 different animals were analyzed to determine each value.
In situ hybridization (ISH)ISH was performed as described previously [17]. A 29-base oligodeoxiribonucleotide containing digoxigenin (DIG)-labeled locked nucleic acid (LNA) (cAggTggAcaAtaGcaGttcG; large capitals represent LNA) was purchased from Gene Design Inc (Ibaraki, Japan) and used as the antisense probe for α-amylase mRNA. The melting temperature (Tm) of this LNA probe was predicted as 76°C using the LNA Tm prediction tool, which can be accessed at https://www.exiqon.com/oligo-tools. The antisense and sense LNA probes without DIG labels were also used. Cryostat sections of the salivary gland specimens were treated successively at room temperature with proteinase K (10 μg/ml) in Tris-EDTA buffer for 10 min, and with 0.25% acetate anhydrate in 0.1 M triethanolamine (pH 8.0) for 10 min, washed in 4× sodium chloride/sodium citrate (SSC), and dehydrated in ethanol. The sections were then incubated with the DIG-labeled antisense probe (10 pmol/l) at 37°C for 15 hr using a hybridization buffer containing 50% deionized formamide, 2× SSC, 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1× Denhardt’s solution, 0.25% SDS, and 200 μg/ml salmon sperm DNA. An excessive amount of an unlabelled antisense or sense probe (1,000 pmol/l) was added to the DIG-labeled antisense probe for the negative control. After hybridization, tissue sections were washed in 0.2× SSC containing 2% bovine serum albumin at 37°C for 5 min. Hybridization signals were detected by incubating the sections successively with an alkaline phosphatase-conjugated anti-DIG antibody (Roche Diagnostics, Basel, Switzerland) diluted at 1:200 in PBS for 1 hr at room temperature and with the substrate Liquid Permanent Red (DAKO, Glostrup, Denmark) in 50 mM Tris-HCl (pH 9.5). ISH results were confirmed in the salivary gland specimens of 3 different animals.
Western blottingFrozen salivary gland tissues were homogenized in lysis buffer containing 25 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and protease inhibitor cocktail (Complete mini; Roche Diagnostics). The tissue homogenates were then separated on 10% SDS-polyacrylamide gels and transferred to PVDF membranes (BioRad Laboratories, Hercules, CA). After being blocked with 0.5% non-fat skimmed milk in 50 mM Tris-HCl (pH 7.5) plus 0.1% Tween20 (TBS-T), the membranes were incubated with a goat polyclonal anti-α-amylase antibody (Sc-12821; Santa Cruz Biotechnologies, Santa Cruz, CA; 1:200 dilution in TBS-T) or a mouse monoclonal anti-α-tubulin antibody (T9026; Sigma, St Louis, MO; 1:1,000) overnight at 4°C. After being washed, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody against goat or mouse IgG (Dako, 1:2,000 in TBS-T) for 1 hr at room temperature. The immunoreaction was visualized and quantified with ImageQuant LAS-4000 mini (Fujifilm Medical, Tokyo, Japan) after the treatment of blots with the chemiluminescent peroxydase substrate ECL-select (Amersham Pharmacia Biotech Inc, Piscataway, NJ).
Immunohistochemistry (IHC)The paraffin sections of salivary gland specimens, after being deparaffinized in xylen and rehydrated in a graded ethanol series, were pre-treated with 3% BSA for 30 min. Sections were incubated overnight at 4°C with a goat polyclonal anti-α-amylase antibody (1:200 dilution in PBS) for IHC by the enzyme-detection method. To confirm the specificity of the immunoreaction, the primary antibody was absorbed with an excess amount (20:1 in weight ratio) of oligopeptide antigen specific for the antibody (Sc-12821p; Santa Cruz Biotechnologies) overnight at 4°C before use. After washing with PBS, immunoreaction sites were visualized by incubating the sections successively with a horseradish peroxidase-conjugated anti-goat IgG antibody (1:200 in PBS) for 1 hr at room temperature, and a peroxidase substrate (ImmPACTTM DAB, Vector Laboratories, Burlingame, CA) for approximately 5 min. The sections were then subjected to observations under an Olympus BX50 microscope. IHC results were confirmed in the salivary gland specimens of 3 different animals.
Statistical analysisStatistical differences were analyzed between two mean values with the Student’s t test, and among multiple mean values with one-factor analysis of variance (ANOVA) followed by Bonferroni’s post hoc test. Differences with a P value less than 0.05 were considered significant.
The expression of α-amylase mRNA in the SMG, SLG, and PG of male and female mice were compared using conventional and quantitative RT-PCR (Fig. 1a, b). The relative levels in the three salivary glands of male mice were approximately 1, 0.1 and 10,000, respectively (P<0.05 between the PG and SMG and between the SMG and SLG). No sexual difference was observed in α-amylase mRNA levels in the PG and SLG, whereas female levels had approximately 30% of male levels in the SMG (P<0.05).
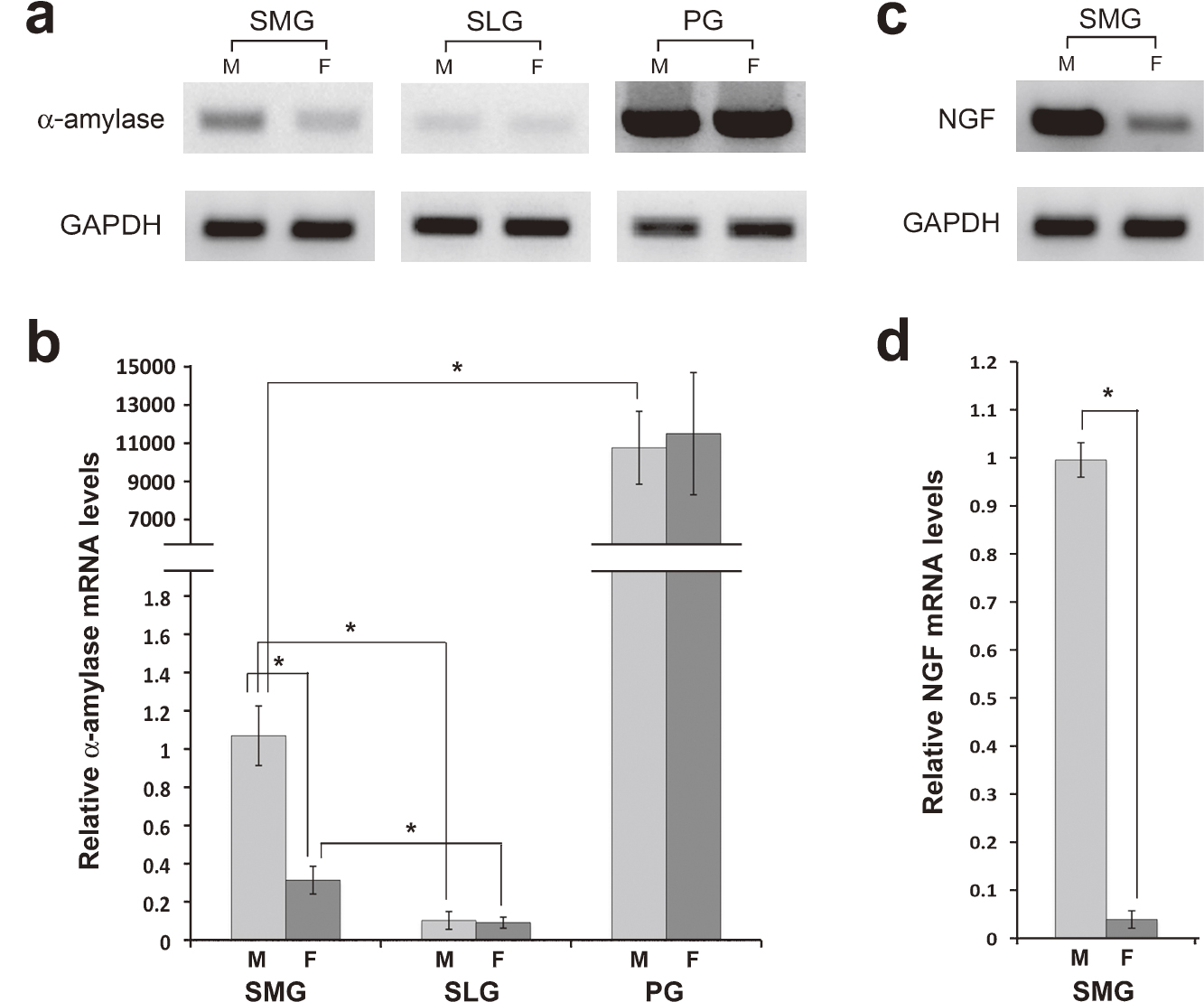
Expression of α-amylase and nerve growth factor (NGF) in the major salivary glands of mice. a. Conventional RT-PCR analysis of the expression of α-amylase mRNA in the submandibular gland (SMG), sublingual gland (SLG), and parotid gland (PG) of male (M) and female (F) mice. The expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was also analyzed as the loading control. After 30 cyles (for α-amylase) or 28 cycles (for GAPDH) of amplification, cDNA fragments were electrophoresed and stained with ethidium bromide. b. Real-time quantitative RT-PCR analysis of the expression of amylase mRNA in the SMG, SLG, and PG of male and female mice. The relative levels of α-amylase mRNA are indicated after normalization with GAPDH mRNA levels. Each value represents the mean±SD of 5 (for SMG) or 4 (for SLG and PG) animals. *Significantly different (P<0.05). c. Conventional RT-PCR analysis of the expression of NGF mRNA in the SMG of male and female mice. The amplification cycle number was 28 for both NGF and GAPDH. d. Real-time quantitative RT-PCR analysis of the expression of NGF mRNA in the SMG of male and female mice. Each value represents the mean±SD of 5 animals. *Significantly different (P<0.05).
We also examined the expression of NGF, a highly specific marker of GCT cells, to determine the extent to which GCT cells were responsible for the sexual dimorphism in α-amylase mRNA levels in the SMG (Fig. 1c, d). NGF transcripts were markedly more abundant in the male than in the female SMG. Quantitative RT-PCR confirmed that female NGF mRNA levels were only 4% of male levels, which can be regarded as proportional to the relative number of GCT cells in the SMG of both sexes.
Localization of α-amylase mRNA in the major salivary glandsISH in the male SMG demonstrated that the cytoplasmic signal for α-amylase mRNA was strong in GCT cells and weaker in acinar epithelial cells (Fig. 2a). ID cells also had a signal similar in intensity to that in acinar cells, but ED cells had a markedly weaker signal. Negative control sections treated with the DIG-labeled antisense probe mixed with an excessive amount of the unlabeled antisense or sense probe had no signal at all (Fig. 2b). Although the signal in acinar cells shown in Figure 2a was weak, it was stronger than that of the negative control shown in Figure 2b. The intensity of the α-amylase mRNA signal in acinar cells in the female SMG was similar to that in the male SMG (Fig. 2c). ID cells and SD cells were also weakly positive for the signal. In the SLG, a moderately strong signal for α-amylase mRNA was localized in cells situated at the distal end of individual acini, which corresponded to serous demilune cells, whereas mucous acinar cells and duct cells had almost no signal (Fig. 2d). In the PG, all acinar cells had a very strong signal for α-amylase mRNA, whereas cells of the duct portions had a markedly weaker signal (Fig. 2e).

In situ hybridization (ISH) analysis of the expression and localization of α-amylase mRNA in the male SMG (a, b), female SMG (c), male SLG (d), and male PG (e) of mice. An excess amount of a non-labeled sense probe was added in b for the negative control. a. A strong signal for α-amylase mRNA was localized in the basal portions of granular convoluted tubule cells (G). Acinar cells (A) were also weakly positive, whereas excretory duct cells (E) were almost negative for the signal. b. No signal was detected in any cells. c. Most of the epithelial cells, including acinar and striated duct cells (S), were weakly positive for the signal. d. Serous demilune cells (D) of acini were moderately positive, whereas mucous acinar cells (M) were almost negative for the signal. e. Acinar cells were strongly positive, whereas intercalated duct (I) and excretory duct (E) cells were almost negative for the signal. Bars=100 μm (a, b, c, e) or 50 μm (d).
Western-blot analysis revealed a single immunoreactive band of 53 kDa that corresponded to α-amylase in all major salivary glands, with higher intensity in the order of PG, SMG and SLG in the male glands. Immunoreactive bands were markedly weaker in the female SMG than in the male SMG (Fig. 3).

Western-blot analysis of α-amylase protein expression in the major salivary glands of mice. Cell lysates of the submandibular gland (SMG), sublingual gland (SLG), and parotid gland (PG) of male (M) and female (F) mice were electrophoresed, blotted, and immunostained with a goat anti-α-amylase antibody. Staining with a mouse anti-α-tubulin antibody was also performed for the loading control. The molecular weights (kDa) of the immunoreactive bands are indicated.
IHC in the male SMG exhibited strong α-amylase immunoreactivity in the secretory granules of GCT cells (Fig. 4a). Reactivity in acinar and ID cells was markedly weaker than that in GCT cells, but was stronger than that in acinar and ID cells of the negative control sections, which were treated with the antibody preabsorbed with the corresponding oligopeptide (Fig. 4b). The intensity of immunoreactivity in acinar and ID cells in the female SMG was similar to that in the male SMG (Fig. 4c). The immunoreactivity in SD cells in the female SMG was markedly weaker than that in GCT cells in the male SMG, but was stronger than that in the male and female acinar cells. In the SLG, moderately strong immunoreactivity for α-amylase was detected in the cells situated at the distal end of individual acini, which corresponded to serous demilune cells, whereas mucous acinar cells and duct cells had almost no reactivity (Fig. 4d). In the PG, all acinar cells exhibited very strong immunoreactivity for α-amylase, whereas cells of the duct portions had markedly weaker reactivity (Fig. 4e).
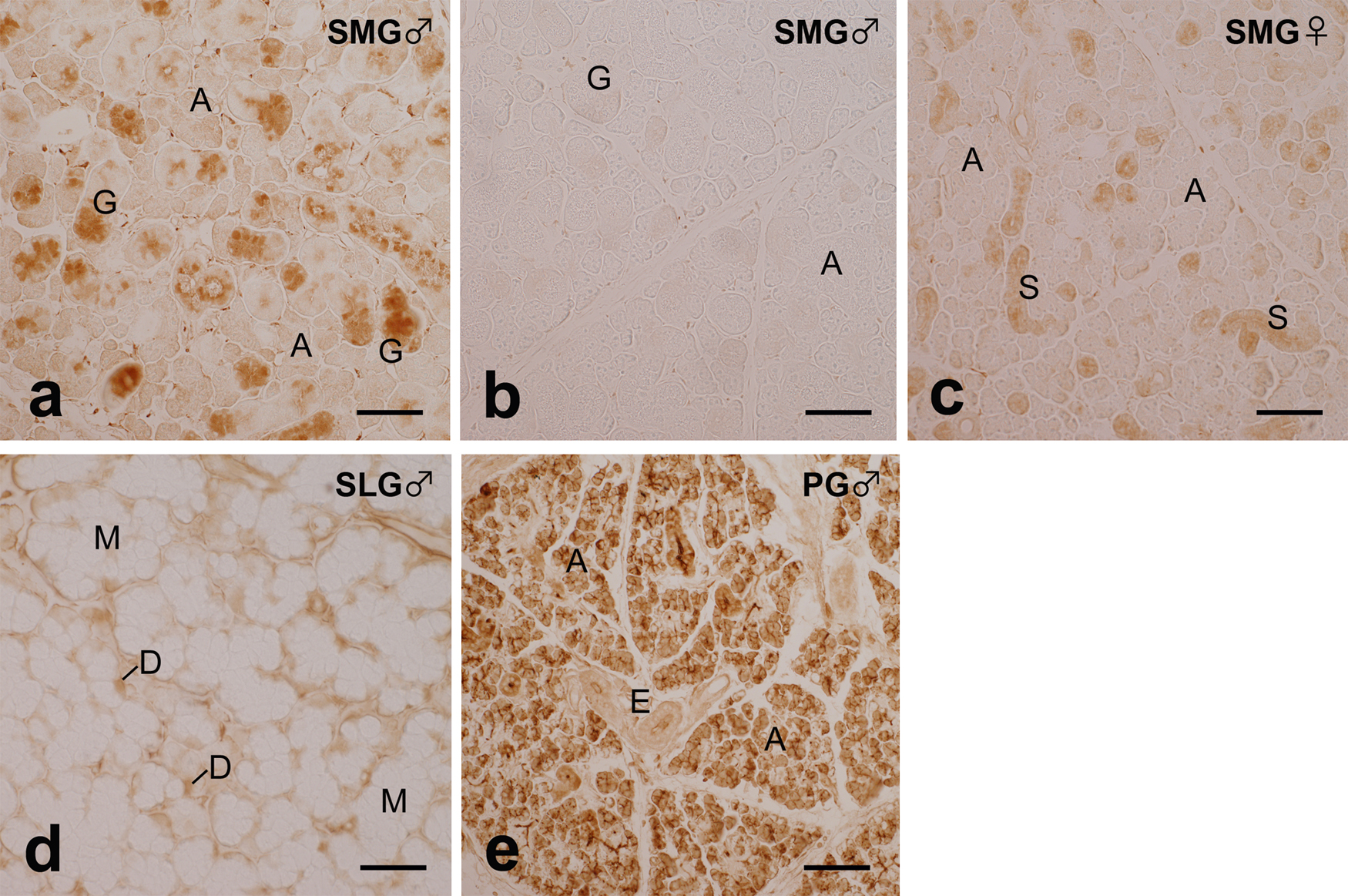
Immunohistochemical analysis of the expression and localization of α-amylase protein in the male SMG (a, b), female SMG (c), male SLG (d), and male PG (e) of mice. The primary antibody was replaced with non-immune goat serum in b for the negative control. a. Strong immunoreactivity for α-amylase was localized in the secretory granules of granular convoluted tubule cells (G). Acinar cells (A) were also weakly positive for the immunoreaction. b. No immunoreactivity was detected in any cells. c. Acinar cells were weakly positive and striated duct cells (S) were moderately positive for the immunoreaction. d. The serous demilune cells (D) of acini were moderately positive, whereas mucous acinar cells (M) were almost negative for the immunoreaction. e. Acinar cells were strongly positive, whereas excretory duct cells (E) were almost negative for the immunoreaction. Bars=100 μm (a, b, c, e) or 50 μm (d).
Quantitative RT-PCR analysis in the present study revealed that α-amylase mRNA expression levels in the major salivary glands of mice were approximately 10,000-fold higher in the PG than in the SMG, and 10-fold higher in the SMG than in the SLG. This is consistent with previous findings on the catalytic activity of α-amylase in the major salivary glands of mice [27]. A strong signal for α-amylase mRNA and immunoreactivity of α-amylase was localized in acinar cells in the PG, which is also consistent with previous immunohistochemical findings in the human [11], rat [26] and mouse PG [27]; however, to the best of our knowledge, ISH results have only been reported for the human PG [15].
In the SMG, the relative levels of α-amylase mRNA expression in females were approximately 30% of those in males, which agrees with previous findings of sexual dimorphism in α-amylase activity in the SMG [7]. ISH further demonstrated that a strong signal for α-amylase mRNA was localized in GCT cells that had developed preferentially in the male SMG, which supports both past and the present results of α-amylase enzyme activity [24, 25, 27] and immunoreactivity [13, the present study] in GCT cells. The reason for the contradiction to some earlier results, which denied the localization of α-amylase immunoreactivity in GCT cells [18, 27], is unknown, but may be attributed to differences in the antibodies used. In addition, the present ISH demonstrated a weaker, but significant mRNA signal in acinar cells of the SMG, with similar intensity being observed in both sexes. Quantitative RT-PCR demonstrated that the relative amount of transcripts for NGF, the specific marker of GCT cells, in females was as low as 4% that in males, in contrast with 30% in the case of α-amylase transcripts. Assuming that this 4% represented the relative number of GCT cells in the female to male SMG, it is estimated that approximately 26% of α-amylase transcripts in the male SMG represent the contribution of acinar cells. Thus, although the present conclusion that GCT cells are the main site of α-amylase production in the mouse SMG has already been suggested, the present study, by analyzing the expression of α-amylase mRNA both quantitatively and histochemically, provided evidence not only for α-amylase production in both GCT and acinar cells, but also for the relative contribution of the two cell populations to α-amylase production.
When the results of ISH and IHC were compared, the difference in intensity of the immunoreactivity between GCT and acinar cells in the male SMG appeared larger than that of the mRNA signal. This is presumably because of the continuous accumulation of α-amylase protein in the secretory granules of GCT cells. Also, the immunoreactivity in the SD portion of the duct system in the female SMG was considerably stronger than that in acinar cells, whereas the difference was not apparent at the levels of the mRNA signal. This may be attributed to the presence of a small amount of typical GCT cells and SD cells with GCT-type secretory granules known to be present in this duct portion of the female SMG [20].
In the SLG, serous demilune cells were found to be the exclusive site of α-amylase mRNA expression, which was consistent with the present immunohistochemical results and supported previous findings on α-amylase immunoreactivity in the secretory granules of serous demilune cells at the electron-microscopic level [9, 13, 14].
During the prenatal and postnatal development of the major salivary glands of rodents [reviewed in 21], both mucous acinar and serous demilune cells of the SLG similar to those of adults have already been formed by the time of birth. In contrast, seromucous acinar cells of the SMG have not been formed at birth, but develop during the first few postnatal weeks from specific precursor cells that are located in the terminal tubules and have serous secretory granules. Seromucous cells in the adult SMG are similar to serous cells in morphology, but secrete a considerable amount of mucins [19]. GCT cells also develop from SD cells in the duct system of the mouse SMG from the onset of puberty at approximately 3 W to the markedly higher number in males than in females [5]. GCT cells have numerous secretory granules that are serous in nature and contain various bioactive peptides. Serous acinar cells of the PG have not yet been formed at birth, but begin to develop from terminal clusters on the first postnatal day. They are serous in nature from the beginning and continue to develop until adulthood, with the increased expression of secretory products including α-amylase [23]. The molecular mechanisms underlying such diversity in salivary gland development from the common rudiment emerging in the embryonic oral epithelium remain to be elucidated [4].
An inverse relationship has been reported between the content of mucin and sialic acid and the activity of α-amylase among the major salivary glands of adult mice [27]. The present study, together with previous findings, have clarified that α-amylase is produced abundantly in acinar cells of the PG, GCT cells of the SMG, and serous demilune cells of the SLG, all of which are serous cells, and moderately in acinar cells of the SMG, which are seromucous cells, but not in acinar cells of the SLG, which are typical mucous cells. The relative contribution of these diverse cell types appears to account for the diversity observed in the production of α-amylase and/or mucins in the major salivary glands of mice.
This work was supported by a Grant-in-Aid from the Ministry of Education, Culture, Science, Sports and Technology of Japan (#23590231).