2015 Volume 48 Issue 3 Pages 95-101
2015 Volume 48 Issue 3 Pages 95-101
To elucidate the fate of the epithelial root sheath during initial cellular cementogenesis, we examined developing maxillary first molars of rats by immunohistochemistry for keratin, vimentin, and tissue non-specific alkaline phosphatase (TNALP) and by TdT-mediated dUTP nick end labeling (TUNEL). The advancing root end was divided into three sections, which follow three distinct stages of initial cellular cementogenesis: section 1, where the epithelial sheath is intact; section 2, where the epithelial sheath becomes fragmented; and section 3, where initial cellular cementogenesis begins. After fragmentation of the epithelial sheath, many keratin-positive epithelial sheath cells were embedded in the rapidly growing cellular cementum. A few unembedded epithelial cells located on the cementum surface. Dental follicle cells, precementoblasts, and cementoblasts showed immunoreactivity for vimentin and TNALP. In all three sections, there were virtually no cells possessing double immunoreactivity for vimentin-keratin or TNALP-keratin and only embedded epithelial cells showed TUNEL reactivity. Taken together, these findings suggest that: (1) epithelial sheath cells divide into two groups; one group is embedded in the cementum and thereafter dies by apoptosis, and the other survives on the cementum surface as epithelial cell rests of Malassez; and (2) epithelial sheath cells do not undergo epithelial-mesenchymal transition during initial cellular cementogenesis.
Hertwig’s epithelial root sheath derives from the most apical extension of the enamel organ and consists of two cell layers, the inner and outer enamel epithelial cells. The epithelial sheath proliferates and grows apically, inducing dental papilla cells to differentiate into odontoblasts. In mammalian teeth, the epithelial sheath is also closely involved in cementogenesis. As root dentinogenesis advances, the epithelial sheath becomes fenestrated and fragmented. Cells of adjacent mesenchymal tissue or dental follicle migrate to the root surface through gaps in the fragmented epithelial sheath, and then differentiate into cementoblasts to secrete cementum matrices. Epithelial cells or cell clusters from the fragmented epithelial sheath survive as epithelial cell rests of Malassez in the periodontal ligament [7, 8]. This concept is referred to as a classical mesenchymal hypothesis on the basis of the mesenchymal origin of cementoblasts [10].
Recently, many investigators have reported that epithelial sheath cells possess mesenchymal or cementoblastic characteristics both in vitro [1, 21, 24, 31] and in vivo [2, 3, 12, 16, 26]. These findings have led to the hypothesis that some epithelial sheath cells transdifferentiate into cementoblasts by epithelial-mesenchymal transition (EMT). This novel idea, referred to as an alternative epithelial hypothesis [10], is now widely agreed upon, because it can explain why epithelial sheath cells decrease in number during epithelial sheath fragmentation, even though some cells may die by apoptosis. According to the in vitro studies it is likely that epithelial sheath cells undergo EMT when cultured under certain conditions. The in vivo studies, however, are considerably questionable in the identification of cementoblasts and epithelial cells.
We agreed with the classical mesenchymal hypothesis in our previous reports, which focus on acellular cementogenesis [28–30]. The present study was designed to examine developing cellular cementum in rat molars by immunohistochemistry (IHC) and by TdT-mediated dUTP nick end labeling (TUNEL), to elucidate the fate of Hertwig’s epithelial root sheath, in particular, whether epithelial sheath cells undergo EMT and how many epithelial sheath cells die by apoptosis during initial cellular cementogenesis. For IHC, keratin, vimentin, and tissue non-specific alkaline phosphatase (TNALP) were used as markers for epithelial cells (keratin), mesenchymal cells (vimentin), and mineralization-inducing cells (TNALP) [9, 13, 14].
Twenty 35-day-old male Wistar rats, weighing 60–70 g, were used in this study. The rats and tissue specimens were treated in accordance with the guidelines of Hokkaido University’s Experimental Animal Committee (No. 10-0081).
After anesthesia with an intraperitoneal injection of sodium pentobarbital, animals were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 15 min. The maxillae were removed, freed of soft tissues, and demineralized in 5% ethylene-diaminetetraacetic acid. Specimens were dehydrated in a graded series of ethanol and embedded in paraffin. Sagittal serial sections of the first maxillary molar were then cut at 5-μm thickness. Some sections were stained with hematoxylin and eosin (H&E) for general histological examination, and others were used for IHC and TUNEL as described herein.
IHC for keratinDeparaffinized sections were immersed in methanol containing 0.3% hydrogen peroxide to inhibit endogeneous peroxidase, and treated with 0.5% trypsin in 0.01 M Tris-HCl buffer (pH 7.6), for 20 min at 37°C. Pre-treated sections were incubated with an anti-pan keratin rabbit polyclonal antibody (Nichirei Co., Tokyo, Japan), followed by an anti-rabbit IgG goat polyclonal antibody conjugated with horseradish peroxidase (HRP) (Nichirei Co.). The immunoreaction was visualized with 3,3'-diaminobenzidine (DAB) as a substrate. Immunostained sections were counterstained with methyl green.
IHC for vimentin and double IHC for vimentin-keratinAfter inhibition of endogenous peroxidase, sections were incubated with an anti-vimentin mouse monoclonal antibody, followed by an anti-mouse IgG goat polyclonal antibody conjugated with HRP (Nichirei Co.), and visualized by DAB method. Sections were counterstained with methyl green, mounted with glycerin, and imaged. Sections were then processed for vimentin-keratin double staining.
After removal of glycerin, sections were treated with trypsin and incubated with an anti-pan keratin antibody (Nichirei Co.), followed by an anti-rabbit IgG secondary antibody, as described above. Keratin immunoreactivity was visualized with the Vector VIP substrate Kit (Vector Laboratories, Burlingame, CA, USA). Double-immunostained sections were counterstained with methyl green.
IHC for TNALP and double IHC for TNALP-keratinAfter inhibition of endogenous peroxidase, sections were incubated with an anti-TNALP rabbit polyclonal antibody produced by Oda et al. [20], followed by an anti-rabbit IgG secondary antibody, for posterior visualization by DAB method. Sections were counterstained with methyl green, mounted with glycerin, and imaged for successive TNALP-keratin double staining.
After removal of glycerin, sections were treated with trypsin and incubated with an anti-pan keratin mouse monoclonal antibody (Abcam, Tokyo, Japan), followed by an anti-mouse IgG secondary antibody. Double-immunostained sections were counterstained with methyl green.
For all sets of IHC experiments, controls were obtained by substitution of normal rabbit or mouse serum for the primary antibodies. These control sections did not show any specific immunoreactivity.
TUNEL assayIn situ Apoptosis Detection Kit (Takara Bio, Otsu, Japan) was used in accordance with the manufacturer’s instructions. Deparaffinized sections were treated with proteinase K (20 μg/mL in phosphate-buffered saline) for 20 min at room temperature. After inhibition of endogenous peroxidase, the sections were incubated with labeling solution (TdT enzyme 5 μL+labeling safe buffer 45 μL), for 90 min at 37°C. Control sections were incubated with only labeling safe buffer. Sections were then incubated with anti-FITC HRP conjugate for 30 min at 37°C, visualized by DAB method, and counterstained with methyl green. Control sections did not show any specific labeling reaction.
Cellular cementogenesis had just begun on the mesial side of the mesial root of the maxillary first molars at postnatal day 35. The apical section of these sides was used to examine the process of initial cellular cementogenesis (Fig. 1A, B). For descriptive convenience, we divided the initial cellular cementogenesis into three stages, which correspond to three distinct sections: section 1, where Hertwig’s epithelial root sheath is intact; section 2, where the epithelial sheath becomes fragmented; and section 3, where cellular cementogenesis begins.

H&E-stained sections showing a 35-day-old rat maxillary first molar. (A) Full view of the tooth. Asterisk indicates a mesial root. Bar=0.5 mm. (B) Magnification of boxed area in A. Two lines demarcate sections 1 to 3. Arrow and asterisk indicate Hertwig’s epithelial root sheath and cellular cementum, respectively. The intact epithelial sheath bends towards the dental pulp (DP). PL, periodontal ligament; AB, alveolar bone. Bar=50 μm. (C) Section 1. Intact epithelial root sheath (between arrows) demarcates dental follicle (DF) and dental papilla (DP). Bar=10 μm (common in C–E). (D) Sections 1 and 2 are partitioned by the dotted line. In section 1, odontoblasts (black arrow) start to form dentin (asterisk). In section 2, the epithelial sheath becomes fragmented and precementoblasts (white arrows) appear. (E) Section 3. Cementoblasts (white arrows) form the initial cellular cementum (double asterisk). Some cementoblasts are embedded as cementocytes (black arrow). Cells or cellular debris (yellow arrows) are seen in the deep region of cementum.
In section 1 (Fig. 1C), the intact epithelial root sheath consisted of inner and outer enamel epithelial cells, and demarcated dental follicle and dental papilla. Dental follicle cells were small and slender, and arranged parallel to the epithelial sheath. Near section 2 (Fig. 1D), dental papilla cells differentiated into odontoblasts and formed dentin. In section 2 (Fig. 1D), the epithelial sheath had begun to fragment. Dental follicle cells became cytoplasmic rich and large cells, which are generally referred to as precementoblasts [6], and assembled on the dentin surface. At this point, however, it was difficult to make a strict distinction between precementoblasts and epithelial cells in H&E-stained sections. In section 3 (Fig. 1E), precementoblasts differentiated into cementoblasts and formed cellular cementum. Some cementoblasts were embedded as cementocytes in the cementum. Cells or cellular debris were present in the deep region of cementum close to the cemento-dentinal junction.
IHC for keratinIn section 1 (Fig. 2A, B), the intact epithelial sheath stained positive for keratin. In section 2 (Fig. 2B), where the epithelial sheath was disrupted, only a few epithelial cells had migrated away from the dentin surface. At this point, the total number of epithelial cells appeared to be fewer than in the intact epithelial sheath. In section 3 (Fig. 2C), cells or cellular debris (see Fig. 1E) in the deep region of the cementum stained positive for keratin, indicating that they are the progeny of epithelial sheath cells. These embedded epithelial remnants were much more than unembedded epithelial cells, i.e., epithelial cell rests of Malassez.
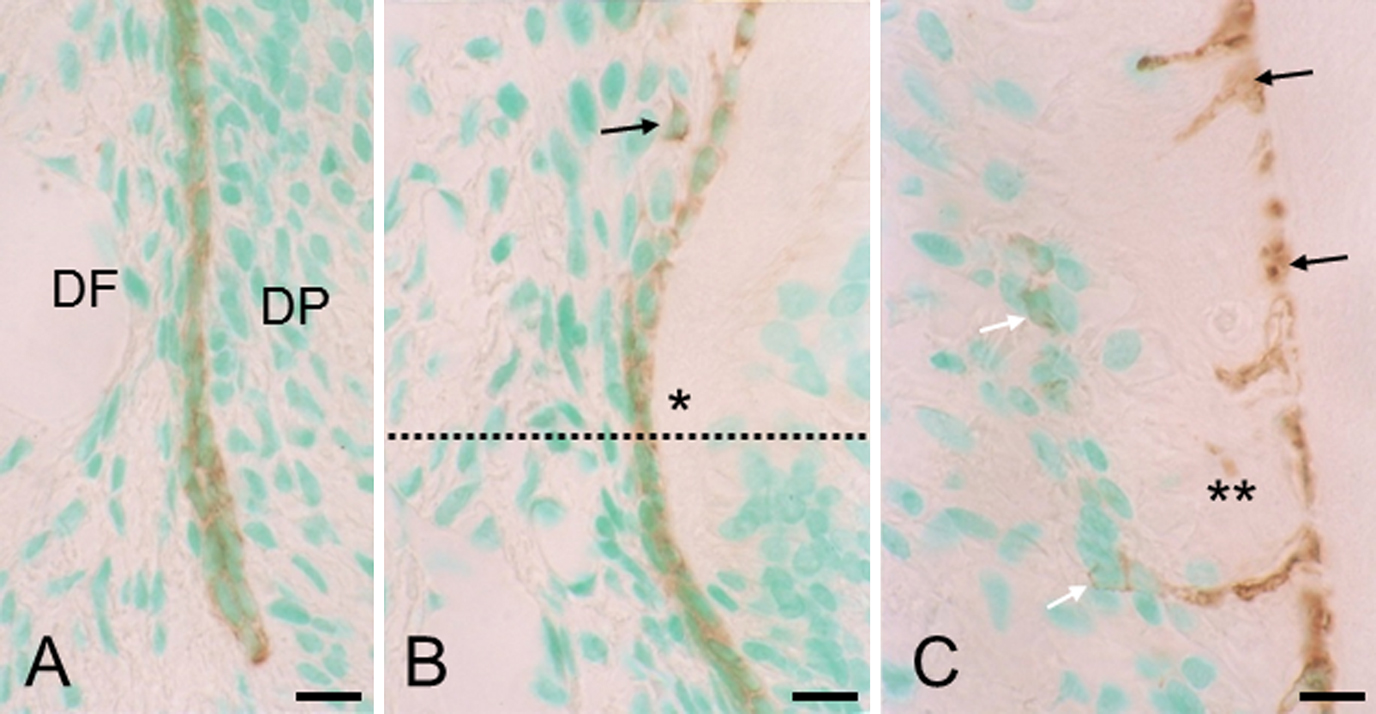
Sections stained for keratin. (A) Section 1. Only intact epithelial root sheath is stained positive for keratin. DF, dental follicle; DP, dental papilla. Bar=10 μm (common in A–C). (B) Sections 1 and 2 are partitioned by the dotted line. In section 2, a few epithelial cells (arrow) are lost from the surface of dentin (asterisk) simultaneously with epithelial sheath fragmentation. The total number of epithelial cells appears smaller than in section 1. (C) Section 3. Many keratin-positive epithelial cells (black arrows) are incorporated in the deep region of cementum (double asterisk), whereas only a few epithelial cells (white arrows) are located on the cementum.
In section 1 (Fig. 3A, B), both dental follicle cells and dental papilla cells stained strongly for vimentin and neither cell type exhibited any keratin immunoreactivity. In contrast, the intact epithelial sheath was only immunoreactive for keratin. Precementoblasts in section 2 (Fig. 3C, D) and cementoblasts in section 3 (Fig. 3E, F) stained strongly for vimentin. Cells embedded in the cementum stained for either vimentin or keratin. In all three sections, no cells on or in the cementum were double immunoreactive for vimentin-keratin.

Sections stained for vimentin (A, C, E) and double stained for vimentin-keratin (B, D, F). (A) and (B), (C) and (D), and (E) and (F) pairs are the same section. Vimentin and keratin stain brown and purple, respectively. Bar=10 μm (common in A–F). (A and B) Section 1. Cells of dental follicle (DF) and dental papilla (DP) stain for vimentin only and epithelial root sheath (between arrows) stains for keratin only. (C and D) Sections 1 and 2 are partitioned by the dotted line. Precementoblasts (black arrows) stain for vimentin only and epithelial cells (white arrows) stain for keratin only. Asterisk indicates dentin. OB, odontoblast layer. (E and F) Cementoblasts (black arrows) stain for vimentin only. Cells incorporated in the cementum (double asterisk) stain for either vimentin (yellow arrow) or keratin (white arrows). From sections 1 to 3, no cells show double immunostaining.
In section 1 (Fig. 4A), the intact epithelial root sheath was negative or only negligibly stained for TNALP. In contrast, the dental follicle, particularly in the alveolar bone-related zone, was stained strongly. In section 2 (Fig. 4A), the intense TNALP immunoreactivity extended towards the root-related side, and in section 3 (Fig. 4B), the entire periodontal ligament, including cementoblasts, exhibited intense TNALP immunoreactivity, whereas cementocytes were negative. At high magnification of section 2 (Fig. 4C, D), dental follicle cells and precementoblasts showed TNALP immunoreactivity on the cell surfaces, and none of these cells were stained for keratin. Keratin-positive epithelial cells showed no or only negligible immunoreactivity for TNALP. Hence, cells possessing double-immunoreactivity for TNALP-keratin were virtually absent. In section 3, TNALP-positive cementoblasts did not show immunoreactivity for keratin (data not shown).

Sections stained for TNALP (A, B, C) and double stained for TNALP-keratin (D). (C) and (D) are the same section. TNALP and keratin stain brown and purple, respectively. (A) Two dotted lines demarcate sections 1 to 3. In section 1, the dental follicle (DF), particularly in the alveolar bone (AB)-related zone, stains intensely for TNALP. The intact epithelial sheath (arrow) shows no immunoreactivity. In section 2, the intense immunoreactivity extends to the root-related side. Asterisk indicates dentin. DP, dental papilla; OB, odontoblast layer. Bar=40 μm (common in A, B). (B) Section 3. The entire periodontal ligament (PL) shows immunoreactivity for TNALP. Double asterisk indicates cellular cementum. (C and D) Magnification of sections 1 and 2 partitioned by the dotted line. Keratin-positive epithelial cells (white asterisks) show no or only negligible immunoreactivity for TNALP. Dental follicle cells and precementoblasts (black asterisks) show TNALP immunoreactivity on the cell surface (black arrows) and do not show any immunoreactivity for keratin. D, dentin. Bars=10 μm.
In sections 1 and 2 (Fig. 5A), no cells showed TUNEL reactivity in the epithelial root sheath or dental follicle. In section 3 (Fig. 5B), embedded epithelial cells had begun to show TUNEL reactivity, and where the cementum formed thick layers (Fig. 5C), many embedded epithelial cells showed TUNEL reactivity in the deep region of cementum.

Sections stained by TUNEL method. (A) Sections 1 and 2 are partitioned by the dotted line. Asterisk indicates dentin. DF, dental follicle; OB, odontoblast layer. Bar=10 μm (common in A–C). (B) Section 3 where cellular cementum (double asterisk) starts to form. (C) Section 3 where cellular cementum (double asterisk) forms thick layer. From sections 1 to 3, only embedded epithelial cells (arrows, see Fig. 2C) show TUNEL reactivity.
The epithelial cells decrease in number during epithelial sheath fragmentation in histological sections [8, 15, 19, 22, 30]. Apoptosis is one possible mechanism for the reduction in cell numbers. In our present study, only embedded epithelial cells showed TUNEL reactivity in section 3. No epithelial sheath cells were TUNEL positive in section 1 or 2. These findings imply that epithelial sheath cells do not die by apoptosis during epithelial sheath fragmentation, thereby excluding apoptosis from the possible reasons for the cell number reduction. This is in agreement with previous studies dealing with apoptosis of the epithelial root sheath [4, 15, 22, 23]. Our present study dealt with initial cellular cementogenesis, in which epithelial cell rests of Malassez are still alive on the cementum surface. Previous studies suggested that the surviving epithelial cell rests also die gradually by apoptosis during further root formation [5, 25].
EMT has been highlighted as another possible mechanism for the cell number reduction. EMT occurs in various organs of normally developing embryos. The EMT in palatogenesis has been often suggested to be comparable to that in cementogenesis, because both palatal epithelium and epithelial root sheath belong to the oral epithelium, and the palatal mesenchyme, like dental follicle, is believed to be of neural crest origin [2]. As palatal fusion progresses, median epithelial cells assume a bipolar fibroblastic appearance and transiently co-express epithelial (keratin) and mesenchymal (vimentin) markers, with the former epithelial cells losing their epithelial characteristics and finally transdifferentiating into mesenchymal cells (fibroblasts) [9]. In this study, we focused on the fact that transforming epithelial cells co-express keratin and vimentin. In addition, TNALP was used as a marker for mineralization-inducing cells or cementoblasts. It has been established that cementoblasts, like other mineralization-inducing cells, such as odontoblasts and osteoblasts, show TNALP activity on the cell membrane [14, 28, 30]. In our study, cells double immunoreactive for vimentin-keratin or TNALP-keratin were virtually absent from all three sections. This implies that EMT does not occur in rat cellular cementogenesis and supports the classical mesenchymal hypothesis. Regarding acellular cementogenesis, our previous reports are consistent with the classical mesenchymal hypothesis [29, 30]. Hirata and Nakamura [11] also questioned the alternate epithelial hypothesis in mouse molars by keratin-osteopontin and -bone sialoprotein double immunostaining. We believe that the classical mesenchymal hypothesis is still convincing in both acellular and cellular cementogenesis.
Here previous studies that support the alternative epithelial hypothesis will be briefly discussed. The in vitro studies reported that: cultured epithelial sheath cells transform into cementoblastic phenotype, expressing cementum-associated transcripts, such as bone sialoprotein and osteopontin, and exhibit a high level of TNALP activity and produce a mineralized cementum-like matrix [1, 21, 24, 31]. We admit that cultured epithelial root sheath cells may undergo EMT under certain conditions. The in vivo studies, however, were based on misidentification of cell types. In most of these studies, investigators used mouse and rat teeth [12, 16, 26]. They found epithelial characteristics in some cementoblasts and cementocytes, thereby proposing that a select population of cementoblasts derive from the epithelial root sheath in acellular and/or cellular cementogenesis. In rat and mouse cementogenesis, however, epithelial cell rests of Malassez remain in the vicinity of the cementum surface and many epithelial sheath cells, as shown in our study, are incorporated in the cellular cementum [4, 17–19, 27]. The other investigators seem to have misidentified the epithelial cell rests and incorporated epithelial cells as cementoblasts and cementocytes, respectively. Taken together, there is no evidence to support the alternative epithelial hypothesis in in vivo cementogenesis.
It is still open to discussion why the epithelial cells decrease in number during epithelial sheath fragmentation, while neither EMT nor apoptosis occurs in the epithelial root sheath. According to Diekwisch [8], the growth rate of the root is much higher than that of epithelial root sheath, and this disproportionate growth rate may explain why very few epithelial cells cover the root surface. In addition to the disproportionate growth rate, we hypothesized that the shape of the epithelial sheath is associated with the reduced cell number in rat acellular cementogenesis [30]. Briefly, the intact epithelial sheath bends towards the dental papilla and forms a tapered cylinder (see Fig. 1B). During root formation the intact epithelial sheath maintains its tapered shape. Under this situation, the discrepancy in surface area could be generated between the epithelial sheath and the developing root. This discrepancy may cause a dispersion of epithelial sheath cells, and accordingly, the reduced cell number appears in histological sections, although the actual number is unchanged. This hypothesis can probably be applied to cellular cementogenesis as well.
In conclusion, regarding the fate of Hertwig’s epithelial root sheath during initial cellular cementogenesis in rat molars, we propose that: (1) epithelial sheath cells divide into two groups; one group is embedded in the cementum and thereafter dies by apoptosis, and the other survives on the cementum surface as epithelial cell rests of Malassez; and (2) epithelial sheath cells do not undergo epithelial-mesenchymal transition.
This study was supported in part by a grant from the Japanese Society for the Promotion of Science to T. Yamamoto (No. 22592028)