2016 Volume 49 Issue 6 Pages 171-179
2016 Volume 49 Issue 6 Pages 171-179
Fibromodulin belongs to the family of small leucine-rich proteoglycans (SLRPs), an active component of extracellular matrix. It directly binds collagens to promote fibrillogenesis and also binds transforming growth factor-beta (TGFβ) to antagonize its actions. Our previous studies of rat anterior pituitary gland revealed that fibromodulin is expressed in folliculostellate cells and pericytes. Although our recent study showed that TGFβ2 secreted from folliculostellate cells induces collagen synthesis in pericytes, the involvement of fibromodulin in TGFβ2-mediated collagen regulation has not been studied. The present study examined the effect of TGFβ2 on fibromodulin synthesis in rat anterior pituitary gland. In situ hybridization for TGFβ receptor II and immunohistological techniques revealed the presence of TGFβ receptor II in folliculostellate cells and pericytes. To confirm canonical TGFβ intracellular signaling, Smad2 immunocytochemistry was performed. Nuclear translocation of Smad2 was observed in folliculostellate cells and pericytes after TGFβ2 treatment. TGFβ2 strongly enhanced fibromodulin mRNA and protein expressions, and TGFβ2-induced mRNA expression was completely blocked by TGFβ receptor I inhibitor (SB431542). These results suggest that folliculostellate cells and pericytes exhibit canonical TGFβ2 signaling, which is associated with fibromodulin production. Thus, this is the first report to show that TGFβ signaling regulates the endogenous TGFβ antagonist fibromodulin in the gland.
Extracellular matrix (ECM) is a complex of collagens, laminins, proteoglycans, and other soluble proteins. These ECM components function as mechanical scaffold and physical barrier and also interact with neighboring cells to exert biological actions on cell proliferation, differentiation, and migration (for comprehensive reviews see Gelse et al. [6] and Paez-Pereda et al. [18]). The small leucine-rich proteoglycan (SLRP) family is a major group of proteoglycans comprising 17 members. SLRPs are differentially expressed in various tissues [22] and are associated with collagen scaffold formation and the ligand-induced cellular signaling pathways [3, 11, 14, 17, 24].
The anterior pituitary gland is composed of hormone-secreting cells and non-hormone-secreting cells, including folliculostellate cells and the cells of capillaries (endothelial cells and pericytes). ECM components in the anterior pituitary communicate with these cells and influence the behavior and biological processes of cells within the gland [9]. At a minimum, they participate in controlling hormone synthesis and secretion [19]. We previously showed that folliculostellate cells and pericytes play an important role in the production of ECM components such as SLRPs and collagens in rat anterior pituitary gland. Decorin, biglycan, fibromodulin, lumican, proline and arginine rich end leucine rich repeat protein (PRELP), and osteoglycin are major SLRPs expressed in the gland [10]. Interestingly, all SLRP-expressing cells in the gland are either folliculostellate cells or pericytes. Folliculostellate cells express various paracrine/autocrine factors [1], whereas pericytes are mural cells of capillaries and are the sole collagen-producing cells in rat anterior pituitary gland [5]. We recently identified a novel cell-to-cell interaction between these cells and discovered that transforming growth factor-beta 2 (TGFβ2) secreted from folliculostellate cells strongly induced collagen synthesis in pericytes [28].
Among the SLRPs, fibromodulin functions as a potent endogenous antagonist of TGFβ by direct binding [7]. Fibromodulin also binds collagens to orient collagen fibrils and is crucial in collagen assembly [3, 24]. In rat anterior pituitary gland, we previously found that the presence of collagen induces fibromodulin mRNA and protein expressions [25]. Taken together, these findings may suggest that fibromodulin, TGFβ2, and collagen regulation are interconnected in anterior pituitary gland. In this study, we investigated whether TGFβ signaling is associated with fibromodulin expression in folliculostellate cells and pericytes by using in situ hybridization for TGFβ receptor, immunocytochemistry for intracellular signal transduction in a monolayer culture of anterior pituitary cells, and a three-dimensional (3D) cell culture method that was successfully developed for our recent study [27].
Wistar rats were purchased from Japan SLC (Shizuoka, Japan). S100β-green fluorescent protein (S100β-GFP) transgenic rats [12], which express GFP under control of the promoter of the S100β protein (a marker of folliculostellate cells) gene, were supplied by the National BioResource Project for the Rat in Japan, Kyoto University (Kyoto, Japan) and bred in our laboratory. Eight- to 10-week-old male rats weighing about 250 g were used. The animals were given ad libitum access to food and water and housed under conditions of 12 hr light and 12 hr darkness. All animal experiments were performed after receiving approval from the Institutional Animal Experiment Committee of Jichi Medical University and were conducted in accordance with the Institutional Regulations for Animal Experiments and Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions, under the jurisdiction of the Japanese Ministry of Education, Culture, Sports, Science and Technology.
In situ hybridizationUnder deep pentobarbital anesthesia, Wistar rats were sacrificed by exsanguination from the right atrium and then perfused with 4% paraformaldehyde (PFA) in 50 mM phosphate buffer (PB, pH 7.4). Pituitary glands were excised and fixed in the same fixative at 4°C overnight, after which the samples were immersed in PB (pH 7.2) containing 30% sucrose at 4°C for 2 days and embedded in Tissue-Tek OCT compound (Sakura Finetechnical, Tokyo, Japan). Frozen sections (8 μm) were obtained using a cryostat (CM3000; Leica Microsystem, Wetzlar, Germany) and mounted on glass slides. In situ hybridization was performed with digoxigenin (DIG)-labeled cRNA probes, as described by Fujiwara et al. [4]. The DNA fragments of rat TGFβ receptor II (Tgfbr2; NM_031132) were amplified from rat pituitary cDNA by PCR with gene-specific primers (Table 1). Amplified DNA fragments were ligated into the pGEM-T vector (Promega, Fitchburg, WI, USA) and cloned. A Roche DIG RNA labeling kit (Roche Diagnostics, Penzberg, Germany) was used to generate gene-specific antisense or sense DIG-labeled cRNA probes. The hybridization was performed at 55°C overnight, and alkaline phosphatase-conjugated anti-DIG antibody (Roche Diagnostics) using 4-nitroblue tetrazolium chloride (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP, Roche Diagnostics) was used for the detection of mRNA. In a control experiment, no specific signal was detected in sections processed with the DIG-labeled sense RNA probes.
| Gene name | Symbol | GenBank Acc. # | Forward sequence (5'-3') | Reverse sequence (5'-3') | size (bp) | Use |
|---|---|---|---|---|---|---|
| TGFβ receptor-II | Tgfbr2 | NM_031132 | GGCGAGACCTTCTTCATGTG | TGTCCTTCTCCGTTTTCCAC | 490 | cRNA probe |
| Fibromodulin | Fmod | NM_080698 | CCACAATAAGATGGGGAAGA | GCACAGATCATGTTGGTCAG | 92 | real-time PCR |
| β-actin | Actb | NM_031144 | TGGCACCACACTTTCTACAATGAGC | GGGTCATCTTTTCACGGTTGG | 106 | real-time PCR |
For double staining, subsequent immunohistochemistry was performed as described previously [4]. The sections were incubated with blocking solution containing 2% normal goat serum (NGS) in phosphate-buffered saline (PBS) for 20 min at room temperature and then with primary antibody for 90 min at 30°C. Primary antibodies included rabbit polyclonal anti-S100 protein (1:1000, DAKO, Glostrup, Denmark) and anti-desmin (1:1000, Abcam, Cambridge, UK). The sections were then incubated with biotinylated anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA) for 30 min at 30°C, and the immunoreactive signal was detected with a Vectastain ABC kit (Vector Laboratories) and 3,3'-diaminobenzidine (Dojindo Laboratories, Kumamoto, Japan).
Cell cultureMonolayer primary cell culture using anterior pituitary cells from Wistar or S100β-GFP transgenic rats was performed as described previously [8]. Dispersed anterior pituitary cells were plated onto glass chamber slides (1 cm2/well; Nalge Nunc Intl., Rochester, NY, USA) at a density of 1×105 cells/well in 400 μl of Medium 199 with Earle’s salts (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA), 0.5 U/ml of penicillin, and 0.5 μg/ml of streptomycin (Thermo Fisher Scientific). Cells were then cultured for 3 days at 37°C in a humidified atmosphere containing 5% CO2.
Hanging drop 3D cell culture using anterior pituitary cells from Wistar rats was performed according to the protocol described previously by Tsukada et al. [27]. A 25-μl drop containing 5,000 anterior pituitary cells was placed on the undersurface of 100-mm Petri dish lids, which were then cultured over sterile PBS at 37°C in a humidified incubator with 5% CO2.
Treatment of TGFβ2 and TGFβ receptor I inhibitor (SB431542)Recombinant human TGFβ2 (PeproTech, Rocky Hill, NJ, USA) and selective TGFβ receptor I inhibitor (SB431542, Merck Millipore, Billerica, MA, USA) were diluted in Hanks’ balanced salt solution (Thermo Fisher Scientific) containing 0.1% bovine serum albumin (BSA) and dimethyl sulfoxide (DMSO), respectively, and stored at −20°C until use. To examine Smad2 nuclear translocation, the cells on a glass chamber slide (2D culture) were treated with TGFβ2 (50 ng/ml) or BSA for 30 min at 37°C in a humidified incubator with 5% CO2. For real-time PCR analysis, 120–150 cell aggregates from Wistar rats were collected in a 15-ml tube 4 days after 3D cell culture and then centrifuged at 200×g for 2 min. Cell culture media were replaced with 470 μl of fresh cell culture media, and all cell aggregates were re-plated onto a 24-well plastic plate. To obtain the indicated concentrations, 30 μl of TGFβ2 and/or SB431542 diluted by cell culture media was added to the well (final volume, 500 μl). The cell aggregates were then incubated for an additional 24 hr at 37°C in a humidified incubator with 5% CO2. BSA and DMSO were diluted in the same manner and used as vehicle controls for TGFβ2 and SB431542, respectively. For Western blot analysis, dispersed anterior pituitary cells from Wistar rats were re-suspended in media containing TGFβ2 (50 ng/ml) or BSA and then cultured in hanging drops (120–150 drops/treatment) for 5 days at 37°C in a humidified incubator with 5% CO2.
Immunofluorescence microscopyTo analyze the nuclear translocation of Smad2, cells treated with TGFβ2 and BSA were fixed in 4% PFA in 25 mM of PB (pH 7.4) for 20 min at room temperature. The cells were permeabilized in PBS containing 0.2% Triton X-100 (Sigma-Aldrich) for 20 min and then incubated in PBS containing 2% NGS for 30 min at room temperature. The cells from S100β-GFP transgenic rats were incubated with rabbit monoclonal anti-Smad2 antibody (1:200, Cell Signaling Technology, Danvers, MA, USA), and the cells from Wistar rats were incubated with rabbit monoclonal anti-Smad2 and mouse monoclonal anti-desmin (1:50, DAKO) antibodies, for 90 min at 30°C, followed by a secondary antibody containing DAPI (0.5 μg/ml) for 30 min at 30°C. The secondary antibodies were Alexa Fluor 568-conjugated goat anti-rabbit IgG, Alexa Fluor 488-conjugated goat anti-rabbit IgG, and Alexa Fluor 568-conjugated goat anti-mouse IgG antibodies (all 1:200, Thermo Fisher Scientific). Stained cells were subsequently analyzed with an FV1000 confocal laser microscope (Olympus, Tokyo, Japan). Images were processed for presentation using Photoshop CS5 (Adobe Systems, San Jose, CA, USA).
Real-time PCR quantification of mRNA levelsAfter treatment with TGFβ2 and/or SB431542 for 24 hr, the total RNA of cell aggregates was extracted by using an RNeasy mini-kit and an RNase-free DNase set according to the manufacturer’s instructions (Qiagen, Hilden, Germany). cDNA was synthesized using the PrimeScript RT reagent kit (Takara Bio, Shiga, Japan) with oligo-(dT)20 primer (Thermo Fisher Scientific). Quantitative real-time PCR (ABI PRISM 7900HT, Applied Biosystems) was performed using fibromodulin primers (Fmod: NM_080698, Table 1) and SYBR Premix Ex Taq (Takara Bio) containing SYBR Green I. For normalization, β-actin (Actb: NM_031144, Table 1) was quantified. All measurements were made in duplicate, and cycle threshold values were converted to relative gene expression levels by using the 2-(ΔCt sample-ΔCt control) method.
Immunoblot analysisTGFβ2- and BSA-treated cell aggregates were washed in Hanks’ balanced salt solution and then lysed in 2× sample buffer (0.5 mM Tris-HCl, 10% SDS, 2-mercaptoethanol, glycerol, pH 6.8). Cell lysates were then boiled at 95°C for 5 min and incubated on ice for another 5 min. Each protein sample (8 μl/lane) was run on 12% SDS-PAGE and transferred electrophoretically to an Immobilon-P transfer membrane (Millipore, Bedford, MA, USA). The blots were incubated with 5% skim milk in T-TBS (50 mM Tris-HCl, 100 mM NaCl, 0.1% Tween 20, pH 7.4) for 1 hr and probed overnight with rabbit polyclonal anti-fibromodulin antibody (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted in Can Get Signal Solution (Toyobo, Osaka, Japan), followed by incubation for 1 hr with horseradish peroxidase (HRP)-labeled secondary antibodies (Envision+System-HRP, anti-rabbit, Dako). Anti-β-actin antibody (0.1 μg/ml; BioVision. Mountain View, CA, USA) was used as a control.
Immunoreactive bands were visualized by ECL plus Western Blotting Detection Reagents (GE Healthcare, Mississauga, ON, Canada) with an LAS-3000 imaging system (Fujifilm, Tokyo, Japan). The acquired images were analyzed by densitometry using ImageJ software (NIH, Bethesda, MD, USA). The data were normalized with β-actin.
Statistical analysisAll results are presented as mean±SEM. The unpaired Student’s t-test for 2-group comparison or 1-way analysis of variance (ANOVA) followed by Dunnett’s test for multiple comparisons was performed using Prism Version 6 (GraphPad Software, San Diego, CA, USA). A P value of <0.05 was considered to indicate statistical significance.
We previously reported that folliculostellate cells and pericytes express fibromodulin in rat anterior pituitary gland [10]. The present study attempted to determine whether these cell types possess TGFβ receptor. We thus performed in situ hybridization for TGFβ receptor II (Tgfbr2), which, by forming a heteromeric complex with TGFβ receptor I, is associated with canonical TGFβ signal transduction. Figure 1a shows hematoxylin-eosin staining of a rat pituitary section. Tgfbr2-expressing cells were located in the posterior, intermediate, and anterior lobes (Fig. 1b). Tgfbr2-expressing cells were found in parenchymal cells, perivascular cells, and endothelial cells of the anterior lobe (Fig. 1c). No specific signal was detected in a section processed with the DIG-labeled sense cRNA probe for Tgfbr2 (Fig. 1d). The Tgfbr2-expressing cells were further stained for S100 protein (a marker of folliculostellate cells) and desmin (a marker of pericytes). The results of double staining showed that Tgfbr2-expressing cells were co-stained with S100 protein (Fig. 1e) and desmin (Fig. 1f).

Expression of TGFβ receptor II mRNA in folliculostellate cells and pericytes of rat anterior pituitary gland. The first row panels show hematoxylin and eosin staining of a cryosection of rat anterior pituitary gland (a) and in situ hybridization of the TGFβ receptor II antisense probe (b). The second row panels show images of in situ hybridization of TGFβ receptor II (c: antisense, d: sense) in the anterior lobe. TGFβ receptor II-expressing cells were observed in parenchymal cells (arrowhead), perivascular cells (arrows), and endothelial cells (double arrowheads). The third row panels show immunohistochemistry of S100 protein (e) and desmin (f), combined with TGFβ receptor II in situ hybridization. The magnified images of (e) and (f) are shown in (g) and (h), respectively. S100 protein was used as a marker for folliculostellate cells, and desmin was used as a marker for pericytes. Immunohistochemical and in situ hybridization signals are shown in brown and purple, respectively. S100 protein-cells (g: open arrow) and desmin-positive cells (h: open arrowhead) expressed TGFβ receptor II. AL: anterior lobe, IL: intermediate lobe, PL: posterior lobe. Bars=100 μm (a, b, e, and f) and 10 μm (c, d, g, and h). Asterisks: capillary lumen.
To confirm the histochemical findings (Fig. 1), we next investigated nuclear translocation of Smad2 in these cell types. Smad2 is a signaling molecule of TGFβ receptor and is transferred into the nucleus in response to TGFβ. We treated isolated rat anterior pituitary cells with TGFβ2 (50 ng/ml) for 30 min and then stained them for Smad2. In the vehicle-treated cells, Smad2 was diffusely stained in both S100β-GFP-positive and desmin-positive cells (Fig. 2Ab, 2Ac, and Fig. 2Bb, Bc, respectively); however, cells treated with TGFβ2 had intense Smad2 signals in the nucleus (Fig. 2Ad, 2Ae, and Fig. 2Bd, Be, respectively). There was zero Smad2 nuclear translocalization in vehicle-treated cells but more than 90% nuclear localization in TGFβ2-treated cells.
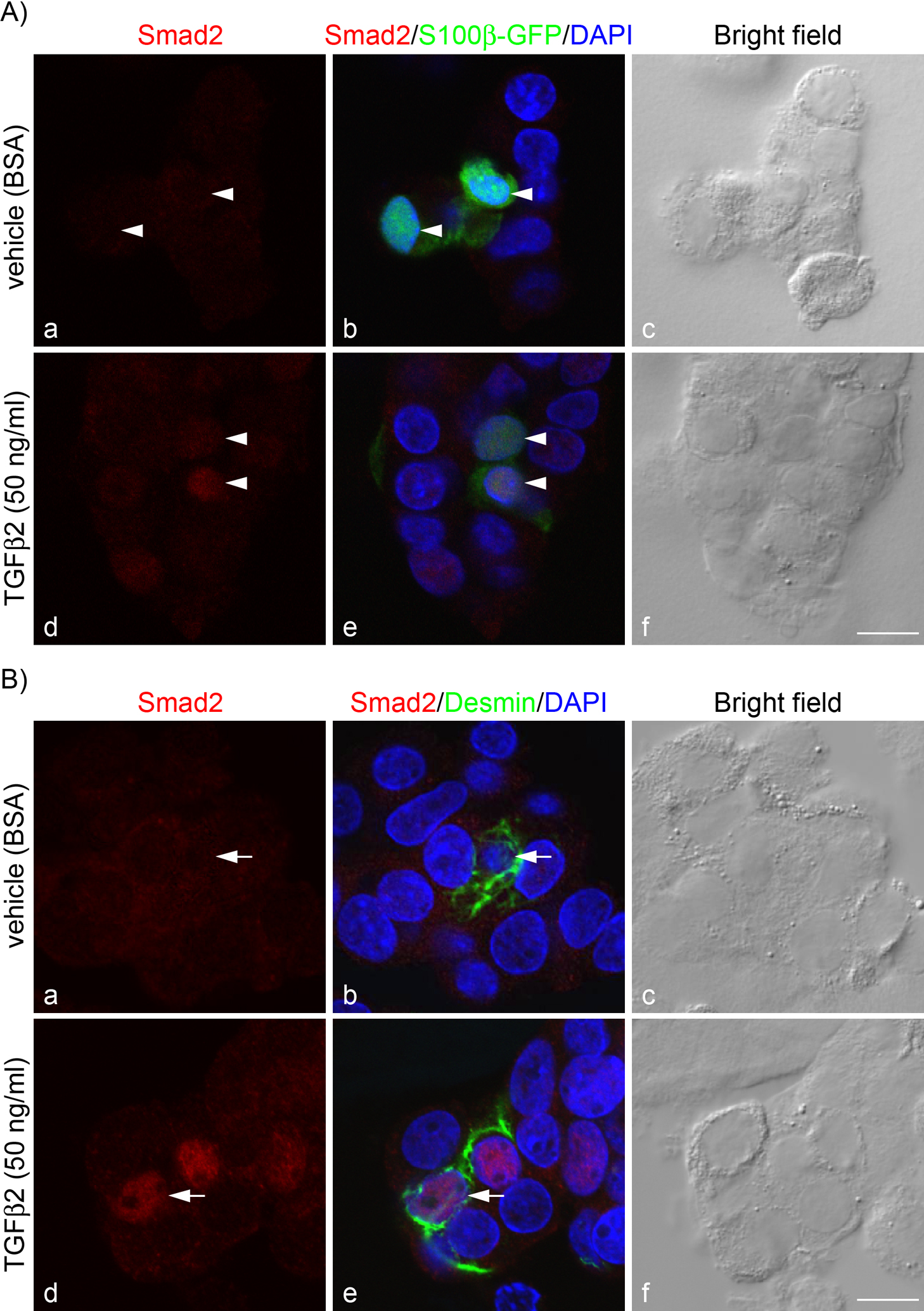
Detection of Smad signaling in folliculostellate cells and pericytes. Anterior pituitary cells from S100β-GFP transgenic rats (A) or Wistar rats (B) were treated with TGFβ2 (50 ng/ml) or 0.1% BSA (vehicle) for 30 min and then stained for Smad2. The left panels show Smad2 staining, the middle panels show a merged image (Smad2: red; S100β-GFP/desmin: green; DAPI: blue), and the right panels show bright-field images. Diffuse cytoplasmic staining of Smad2 was seen in vehicle-treated GFP-positive cells (folliculostellate cells: Aa, Ab) and desmin-positive cells (pericytes: Ba, Bb); however, nuclear staining was seen in TGFβ2-treated GFP-positive cells (Ad, Ae) and desmin-positive cells (Bd, Be). The cells, Smad2 positive in the nucleus and desmin-negative, in Fig. Be are considered to be folliculostellate cell or endothelial cell, because they express TGFβ receptor II [28]. Arrowheads: nucleus of GFP-positive cells. Arrows: nucleus of desmin-positive cells. Bars=10 μm.
To determine whether TGFβ2 affects fibromodulin mRNA synthesis, anterior pituitary cell clusters from Wistar rats were treated with TGFβ2 for 24 hr, and fibromodulin expression was examined by quantitative real-time PCR (Fig. 3). TGFβ2 significantly increased fibromodulin expressions in a dose-dependent manner (Fig. 3A; by approximately 2-, 4-, 8-, and 22-fold for 1, 5, 10, and 50 ng/ml of TGFβ2, respectively). Furthermore, TGFβ2-induced fibromodulin expression was completely abolished by co-administration of TGFβ receptor I inhibitor (Fig. 3B).

Relative mRNA expression level of fibromodulin in cell aggregates treated with TGFβ2 and/or selective TGFβ receptor I inhibitor (SB431542) evaluated by quantitative real-time PCR. Anterior pituitary cells of Wistar rats were used. mRNA expression levels were normalized with β-actin. A) Cell aggregates were treated with different concentrations of TGFβ2 (1–50 ng/ml). TGFβ2 induced fibromodulin synthesis in a dose-dependent manner (n=4, mean±SEM). B) Cell aggregates were treated with TGFβ2 (50 ng/ml) and SB431542 (10 μM). BSA and DMSO were the vehicle control for TGFβ2 and SB431542, respectively. SB431542 attenuated TGFβ2-induced fibromodulin synthesis (n=4, mean±SEM). *P<0.05 (Dunnett’s test).
Because increased fibromodulin mRNA expression was found in cells treated with TGFβ2, we next used Western blot analysis to examine the effect of TGFβ2 on fibromodulin protein expression. In this experiment, anterior pituitary cell clusters from Wistar rats were treated with TGFβ2 for 5 days. Major immunoreactive bands for fibromodulin were detected at approximately 67 kDa, the expected size of fibromodulin with N-linked oligosaccharides. TGFβ2 at a dose of 50 ng/ml significantly increased fibromodulin protein expression, by approximately 3-fold (Fig. 4).

Relative protein expression level of fibromodulin in cell aggregates treated with TGFβ2 evaluated by Western blotting. Anterior pituitary cells of Wistar rats were treated with TGFβ2 (50 ng/ml) for 5 days in hanging-drop culture. A) Top: Fibromodulin (Fmod); bottom: β-actin. B) The graph shows the results of immunoblot analysis as fold induction normalized against β-actin (n=3, mean±SEM). *P<0.05 (Student’s t-test).
The present findings indicate that fibromodulin-expressing cells (folliculostellate cells and pericytes) in rat anterior pituitary gland possess TGFβ receptor II and that both cell types respond to TGFβ2 and increased fibromodulin mRNA and protein expressions. This is the first report to show that fibromodulin known as an endogenous TGFβ antagonist is regulated by TGFβ signaling in anterior pituitary gland.
In situ hybridization revealed the localization of TGFβ receptor II mRNA in rat anterior pituitary gland (Fig. 1c). As was the case in our previous report [28], TGFβ receptor II mRNA was detected in parenchymal cells, perivascular cells, and endothelial cells. Double staining showed that parenchymal cells were co-stained with S100 protein and that perivascular cells were co-stained with desmin (Fig. 1e, f). Because fibromodulin is produced by folliculostellate cells and pericytes in the anterior pituitary gland [10], our results suggest that fibromodulin-expressing cells possess TGFβ receptor II.
TGFβ receptor II forms a heteromeric complex with TGFβ receptor I, which induces intracellular signal transduction, including phosphorylation and nuclear translocation of SMADs (for a review see Macias et al. [16]). To determine whether expressed TGFβ receptor II in fibromodulin-expressing cells is functional, we performed Smad2 immunocytochemistry using rat anterior pituitary cells (Fig. 2). Our results showed intense Smad2 immunoreactivity in the nucleus of S100β-GFP-positive cells and desmin-positive cells after TGFβ2 treatment (Fig. 2Ad and Bd, respectively). These data strongly suggest that both folliculostellate cells and pericytes respond to TGFβ2 and have canonical TGFβ signals.
Our previous study using a 3D cell culture system of rat anterior pituitary cells showed that TGFβ2 acts on pericytes and induces type I and III collagen synthesis [28]. In the present experiment, we used the same technique to evaluate the effect of TGFβ2 on fibromodulin expression. We found that fibromodulin mRNA expression was significantly higher after TGFβ2 treatment and that the action was dose-dependent (Fig. 3A). Consistent with this increased mRNA expression, fibromodulin protein levels, as evaluated by Western blot analysis, were also enhanced by TGFβ2 (Fig. 4). That TGFβ2 increases SLRP fibromodulin expression in anterior pituitary is a novel finding, although some reports noted that TGFβs induce small proteoglycans in other tissues [2, 13, 23, 30]. Furthermore, TGFβ2-induced fibromodulin expression was completely abolished by co-administration of SB431542, an inhibitor of TGFβ receptor I (Fig. 3B). These results suggest that canonical TGFβ signals in folliculostellate cells and pericytes are associated with fibromodulin gene regulation. Further studies are needed in order to clarify the mechanism by which the fibromodulin gene is regulated by TGFβ-induced SMAD signaling in these cells.
TGFβ is a secreted growth factor and has various effects on the biological activities of cells [21]. It is a potent regulator of ECM synthesis, in which signaling stimulates synthesis of ECM components [20, 28, 29]. Indeed, our past and present findings show that TGFβ2 induced fibromodulin and collagen synthesis in pericytes [28]. It is highly likely that fibromodulin and collagen synthesis in pericytes utilizes the same pathway. Because TGFβ2 is exclusively expressed in folliculostellate cells in anterior pituitary gland [28], the action of TGFβ2 on pericytes is probably a paracrine mechanism. Fibromodulin binds different types of collagen, stimulates early protofibril assembly, and facilitates maturation of fibrils [3]. Thus, the simultaneous increase in fibromodulin and collagen expressions in pericytes may be important for proper fibrillogenesis in anterior pituitary gland.
In contrast, the action of TGFβ2 on folliculostellate cells is considered to be an autocrine mechanism. In addition to collagen, fibromodulin binds TGFβ and potently sequesters its activity [7]. Increased fibromodulin expression in folliculostellate cells probably modulates the action of active TGFβ by its antagonistic action and functions as negative feedback regulator. Fibromodulin expression in folliculostellate cells is also increased when cells are cultured with collagen and laminin [25]; thus, there is another pathway to regulate fibromodulin expression in folliculostellate cells.
Evidence indicates that local interactions are mediated by ECM within the gland [18]. Studies of the functions of collagen in anterior pituitary gland show that it induces folliculostellate cell proliferation [10] and SLRP synthesis [25], alters cell morphology [26], and promotes hormone production [15]. Because of its variety of actions on anterior pituitary cells, it is probable that collagen is regulated in anterior pituitary. The present study may highlight the importance of interaction between TGFβ signaling and fibromodulin in collagen regulation in anterior pituitary gland.
The authors have no conflict of interest that might prejudice the impartiality of this research.
We thank David Kipler, ELS (Supernatant Communications) for revising the language of the manuscript.
This work was partly supported by JSPS KAKENHI Grants (25860147 to TT), by promotional funds from the Keirin Race of the Japan Keirin Association, and by the Jichi Medical University Young Investigator Award from Jichi Medical University School of Medicine (to TT and MA).