2016 Volume 49 Issue 6 Pages 181-190
2016 Volume 49 Issue 6 Pages 181-190
Gastrin-releasing peptide (GRP) has recently been identified as an itch-signaling molecule in the primary afferents and spinal cord of rodents. However, little information exists on the expression and localization of GRP in the trigeminal somatosensory system other than in rats. We examined the generality of the trigeminal GRP system in mammals using two distinct species, suncus as a model of specialized placental mammals known to have a well-developed trigeminal sensory system and mice as a representative small laboratory animal. We first analyzed the gross morphology of the trigeminal somatosensory system in suncus to provide a brainstem atlas on which to map GRP distribution. Immunohistochemical analyses showed that 8% of trigeminal ganglion neurons in suncus and 6% in mice expressed GRP. Expression was restricted to cells with smaller somata. The GRP-containing fibers were densely distributed in the superficial layers of the caudal part of the trigeminal spinal nucleus (Vc) but rare in the rostral parts, both in suncus and mice. Expression of GRP receptor mRNA and protein was also detected in the Vc of suncus. Taken together, these results suggest that the trigeminal GRP system mediating itch sensation is conserved in mammals.
Itch is defined as “an unpleasant sensation which provokes the desire to scratch” [19]. Scratching induced by itch promotes the release of endogenous pruritogens that exacerbate itch. Consequently, the “itch–scratch cycle” worsens skin damage and dermatitis. It has been reported that itch sensation is transmitted in the spinal cord by small neurons with unmyelinated C-fiber [5, 6, 18, 25, 32]. Until recently, no itch-specific mediator had been identified due to the similarities of this sensation with pain. An important recent finding is that spinal itch transmission is at least partly independent of pain transmission and relies on gastrin-releasing peptide (GRP)/GRP receptor (GRPR) signaling in the spinal dorsal root ganglion (DRG)–dorsal horn of the spinal cord in mice [26]. Scratching behavior is reduced by spinal intrathecal injection of a GRPR antagonist in GRPR null mice [26]. Histological analyses in mice revealed that GRP-immunoreactivity is localized mainly in small- and medium-size neurons of the DRG [3, 26]. Some GRP-immunoreactive (ir) DRG neurons co-expressed with other peptide; e.g., calcitonin gene-related peptide and substance P, and ion channel such as transient receptor potential vanilloid 1 in rodents [1–3, 13, 26, 28, 34]. In the spinal cord, GRP-ir fibers were observed specifically in the spinal dorsal horn laminae I and II outer layers. An atopic dermatitis NC/Nga mouse model of chronic pruritus showed high densities of GRP-containing fibers in the epidermis [30]. The expression levels of GRP in the DRG and GRPR in the spinal dorsal horn were both increased in allergic contact dermatitis and in dry skin mouse models [34]. Similar results were also reported in monkeys and mice with chronic pruritus [2, 15, 24].
Previously, we demonstrated that GRP is expressed in small- and medium-size trigeminal ganglion (TG) neurons as well as in smaller DRG neurons of rats, indicating that a trigeminal GRP system exists in rodents [28]. Cranial TG neurons convey sensation from the orofacial region and terminate in the trigeminal sensory nuclei of the brainstem. The trigeminal sensory nuclei are structurally and functionally divided into the trigeminal sensory nucleus principalis (Vp) and three spinal subnuclei, oralis (Vo), interpolaris (Vi), and caudalis (Vc) [17]. We previously demonstrated that GRP-expressing axons and terminals in the trigeminal sensory system of rats were mainly localized in the superficial laminae of the Vc [28]. These findings imply that the GRP/GRPR system plays an important role in the transmission of itch sensation not only from the trunk and limbs but also from the orofacial region in rodents.
However, little information exists on the somatosensory system mediating itch transmission in animals other than rodents and primates. The Asian house musk shrew Suncus murinus (laboratory name; suncus) of the order Eulipotyphla (formerly Insectivora) is thought to resemble specialized placental mammals (eutherians) [33]. Genetic evidence also indicates that the suncus is more closely related to primates than to rodents [7], and so it may provide a phylogenic bridge between rodent and primate studies. A relatively well developed trigeminal sensory system is observed in suncus [9], possibly to compensate for the relatively degenerate vision system. Therefore, we focused on suncus as a laboratory small animal model that may possess a well developed trigeminal sensory system unique to subterranean eutherians. Recently, we cloned cDNA encoding GRP in suncus (accession number: suncus Grp; LC138361) [29]. The mature GRP in suncus is similar to human rather than rodent GRP. Particularly, both suncus and human GRP proteins share an identical neuromedin C (NMC or GRP-10) region at the C-terminus.
In this study, we examined the generality of the mammalian trigeminal somatosensory GRP/GRPR system by mapping GRP/GRPR expression in a subterranean eutherian, suncus, known to have a well-developed trigeminal sensory system, and in the mouse as a representative small rodent laboratory animal.
The male and female adult suncus (12–52 weeks old) studied here are an outbred KAT strain established from a wild population in Kathmandu, Nepal [4, 10, 31]. They were maintained according to an established procedure [4]. Male adult mice of the C57BL/6J strain (12 weeks old) were purchased from Shimizu Laboratory Supplies Co. (Kyoto, Japan) and maintained under standard conditions. All experimental procedures were approved in accordance with the Guide for the Care and Use of Laboratory Animals prepared by Okayama University (Okayama, Japan), by Okayama University of Science (Okayama, Japan), and by Kyoto Prefectural University of Medicine (Kyoto, Japan) and were performed in accordance with the National Institutes of Health guidelines on animal care. All efforts were made to minimize animal suffering and reduce the number of animals used in this study.
Tissue preparation for histological analysesSuncus (n=4 of each sex) and mice (n=4 males) were deeply anesthetized by intraperitoneal injection of 50 mg/kg body weight sodium pentobarbital and transcardially perfused with physiological saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brainstem, cervical spinal cord, and trigeminal ganglia were removed, immersed in the same fixative for 3 hr at room temperature, and then immersed in 25% sucrose in 0.1 M PB for 48 hr at 4°C. For toluidine blue staining and cryosection immunohistochemistry (IHC), tissues were quickly frozen using powdered dry ice and cut into 30 μm-thick sections on a cryostat (CM3050 S; Leica, Nussloch, Germany). These sections were then washed several times (5 min/wash) with phosphate-buffered saline (PBS).
Toluidine blue staining and IHCEvery fourth section was used for preparation of the brainstem atlas by toluidine blue staining, so the interval between atlas diagrams is 0.12 mm. Sections were mounted in sequence on glass slides and the slides were immersed sequentially for 2 min in 100% ethanol, xylene, 100% ethanol, 90% ethanol, and 70% ethanol. They were then stained with 0.1% toluidine blue in sodium borate buffer for 10 min and dehydrated through a graded ethanol series (70%, 80%, 90%, 100%). The preparations were then soaked in xylene and covered slipped. For immunoperoxidase histochemistry, sections were first incubated with 1% H2O2 in absolute methanol for 20 min to eliminate endogenous peroxidase activity. Sections were then rinsed with PBS three times (5 min/rinse). After blocking nonspecific binding with 1% normal goat serum and 1% bovine serum albumin (BSA) in PBS containing 0.1% Triton X-100 for 1 hr at room temperature, sections were incubated with primary rabbit antiserum against mouse GRP20-29 (11081; Assaypro, St. Charles, MO, USA) for 48 hr at 4°C. This antiserum produced identical patterns of labeling in a population of GRP neurons of the rat somatosensory system as achieved by IHC using the same antiserum [23, 28]. Full details of all antibodies used are presented in Table 1. The specificity of the GRP antiserum reactivity was also confirmed by control absorption experiments. Briefly, the primary rabbit antiserum against GRP (1:2,000 dilution) was preabsorbed with excess suncus GRP18-27 antigen peptide (50 μg/mL: 2[Asn]-NMC; AnaSpec; San Jose, CA, USA) overnight at 4°C before use. Immunolabeling was detected using a streptavidin-biotin kit (Nichirei, Tokyo, Japan) followed by diaminobenzidine development as described previously [28]. Immunoreacted sections were analyzed using an Olympus Optical microscope (Tokyo, Japan). Images were captured by a CCD camera and saved in TIFF format. Digital photomicrographs were processed with Adobe Photoshop CS5.1 at 300 dpi resolution.
| Antigen | Immunogen | Species, types | Dilution used | Catalog information |
|---|---|---|---|---|
| GRP | Mouse neuromedin C (or GRP-10; GSHWAVGHLM) |
Purified rabbit polyclonal IgG | 1:1,000 (IF) 1:2,000 (IHC) |
AssayPro, 11081-05015 |
| GRPR | Synthetic peptide within amino acids 200 and 294 of human GRPR |
Purified rabbit polyclonal IgG | 1:1,000 (WB) | GeneTex, GTX100015 |
Abbreviations: IF, immunofluorescence histochemistry; IHC, immunohistochemistry; GRP, gastrin-releasing peptide; GRPR, gastrin-releasing peptide receptor; WB, Western immunoblotting.
For estimation of the proportion of GRP-ir TG neurons and analyses of TG neuron size distribution in suncus and mice, fluorescent Nissl staining was combined with GRP immunofluorescence (IF). An Alexa Fluor 488-linked anti-rabbit IgG raised in goats (Molecular Probes, Eugene, OR, USA) was used at a 1:1,000 dilution for detection. After staining for GRP, sections were incubated with NeuroTrace Fluorescent Nissl Staining 530/615 (1:100 dilution; N-21482; Molecular Probes) as a neuronal marker. Dual-stained sections were imaged with a LSM510META confocal laser-scanning microscope (Carl Zeiss, Jena, Germany). Confocal images were saved in TIFF format and processed using Zeiss LSM Image Browser software (Carl Zeiss). The numbers and perikaryon sizes of GRP-ir neurons with distinct nuclei were determined in the suncus and mouse TG. The soma size of TG neurons was calculated as the area of an ellipse (π × half the major axis length of the soma × half the minor axis length of the soma). The cross-sectional areas of 6,350 TG neurons were measured in 65 sections from 8 TG of 4 male suncus, 12,479 TG neurons in 91 sections from 8 TG of 4 female suncus, and 7,582 TG neurons in 64 sections from 8 TG of 4 male mice.
Reverse transcription (RT)-PCR for Grpr in the Vc and the cervical spinal dorsal horn in suncusRT-PCR analysis was performed to estimate the expression of Grpr mRNA in the Vc and cervical spinal dorsal horn of suncus (n=3 of each sex). Adult suncus were deeply anesthetized by intraperitoneal injection of 50 mg/kg body weight sodium pentobarbital and sacrificed by blood loss. Dissected tissues (the Vc and cervical spinal dorsal horn) from suncus were immediately fixed with RNAlater (Thermo Fisher Scientific, Waltham, MA, USA) and stored at −80°C until RNA extraction. Total RNA was extracted from samples using the Sepasol-RNA I Super G kit (Nacalai Tesque, Kyoto, Japan) according to the manufacture’s protocol. The RNA concentration was measured using the Qubit RNA assay kit (Thermo Fisher Scientific). First-strand cDNA was synthesized from 100 ng of total RNA with oligo-dT primers using the Omniscript RT Kit (QIAGEN, Hilden, Germany) in a 20-μl reaction volume. The predicted suncus Grpr sequence (accession number in GenBank: suncus Grpr; LC149855) was obtained from an unpublished suncus genome resource. The sequences of primers for RT-PCR analysis were also designed based on this genome resource (see Table 2). The resultant PCR amplicons were electrophoresed on 2% agarose gels. Each RT-PCR study was repeated three times using independently extracted RNA samples from different animals.
| Species | Target gene | Forward primer | Reverse primer | PCR condition (cycle) |
|---|---|---|---|---|
| Suncus | Grpr | TCACCCTGATCAAGATCTTCTGTAC | ACTCTGGATCAGATTTTTGGCAATG | (94°C 30 sec, 60°C 30 sec, 72°C 60 sec) × 35 |
Abbreviation: Grpr, gastrin-releasing peptide receptor.
Western blotting was conducted according to our previously described methods [22]. In brief, adult suncus (n=2 of each sex) were sacrificed by blood loss under deep pentobarbital anesthesia. Brains and spinal cords were quickly removed and placed on ice, and the Vc and cervical spinal dorsal horn were carefully dissected. Samples were snap-frozen immediately in liquid nitrogen and used for Western analysis. Lysate samples for GRPR measurement (100 μg Vc and 50 μg cervical spinal dorsal horn protein) were boiled in 10 μL sample buffer containing 62.5 mM trishydroxymethyl-aminomethane-HCl (Tris-HCl; pH. 6.8), 2% SDS, 25% glycerol, 10% 2-mercaptoethanol, and a small amount of bromophenol blue. Samples were then separated by 4%–20% gradient SDS-PAGE and electroblotted onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA, USA) using a semidry blotting apparatus (Bio-Rad Laboratories). Membranes were blocked with the PVDF Blocking Reagent for the Can Get Signal kit (TOYOBO, Tokyo, Japan) for 30 min at room temperature and then incubated overnight at 4°C in Can Get Signal Solution 1 (TOYOBO) containing a 1:1,000 dilution of rabbit polyclonal antibody against human GRPR (Table 1). Blotted membranes were washed three times with 0.05% Tween 20 in Tris-HCl buffered saline (TBST) and incubated with horseradish peroxidase (HRP)-conjugated goat polyclonal antibody against rabbit IgG (Bio-Rad Laboratories) at a 1:10,000 dilution in Can Get Signal Solution 2 (TOYOBO) for 1 hr at room temperature. After washing five times with TBST, blots were visualized by the Immun-Star WesternC Chemiluminescence Kit (Bio-Rad Laboratories). Images of immunoblots were adjusted slightly for brightness and contrast to provide a uniform background.
Toluidine blue staining was first performed to provide an atlas of male suncus brainstem to map the GRP signaling pathway (Fig. 1). The head of suncus is substantially flattened along the rostro-caudal axis. Consistent with this gross morphology, the suncus brainstem is flattened, especially in the caudal part, whereas the trigeminal ganglion is disproportionately large for the body size. In particular, the spinal trigeminal sensory nuclei (e.g., Vi and Vo) extend massively in the brainstem, indicating well-developed somatosensory receptivity (Figs. 2, 3).

Toluidine blue staining of male suncus brainstem. The interval between sections is 0.12 mm from midbrain to medulla oblongata. Presented from the periaqueductal gray level (rostral) of the midbrain to the caudal medulla in order from the top-left. Bar=1 mm.

Schematic frontal sections illustrating the suncus brainstem. A series of illustrations of the brainstem from the midbrain (A) to cervical spinal cord (G). Neuroanatomy was confirmed by toluidine blue staining. The capital letter on the left side of each drawing indicates the rostrocaudal level of the section as shown on the schema of the external view. Abbreviations in (A): Aq, aqueduct; IC, inferior colliculus; PAG, periaqueductal gray; PRn, pontine reticular nucleus. (B): GR, granular layer; LC, locus coeruleus; LP, Purkinje cell layer; MO, molecular layer; Pr5, principal sensory trigeminal nucleus; RF, reticular formation; s5, sensory root of the trigeminal nerve; 4V, fourth ventricle. (C): RF, reticular formation; sp5, spinal trigeminal tract; SP5O, spinal trigeminal nucleus, oral part; Ve, vestibular nucleus. (D): Amb, ambiguous nucleus; RF, reticular formation; sp5, spinal trigeminal tract; SP5I, spinal trigeminal nucleus, interpolar part; Ve, vestibular nucleus. (E): AP, area postrema; CC, central canal; Cu, cuneate nucleus; py, pyramidal tract; RF, reticular formation; Sol, nucleus solitary tract; sp5, spinal trigeminal tract; SP5C, spinal trigeminal nucleus, caudal part; SP5I, spinal trigeminal nucleus, interpolar part; 10N, dorsal motor nucleus of vagus; 12N, hypoglossal nucleus. (F): CC, central canal; py, pyramidal tract; RF, reticular formation; Sol, nucleus solitary tract; sp5, spinal trigeminal tract; SP5C, spinal trigeminal nucleus, caudal part; 10N, dorsal motor nucleus of vagus. (G): CC, central canal; DH, dorsal horn; VH, ventral horn.

Toluidine blue staining of the trigeminal sensory nuclei and spinal cord in male suncus. (A) Trigeminal sensory nucleus principalis (Vp). (B) Spinal trigeminal nucleus oralis (Vo). (C) Spinal trigeminal nucleus interpolaris (Vi). (D) Transition area between Vi and the spinal trigeminal nucleus caudalis (Vc). (E) Vc. (F) Cervical spinal cord. Bar=1 mm.
Immunohistochemical (IHC) analysis of male suncus revealed GRP-containing fibers in the TG (Fig. 4A) and caudal medulla (Fig. 4B). The distribution pattern of GRP-ir neurons was similar to that seen in rat tissues [28]. Staining was completely abolished in both TG (Fig. 4C) and caudal part of medulla (Fig. 4D) (n=4) when the antiserum was preabsorbed with suncus GRP18-27.

Immunohistochemical staining using gastrin-releasing peptide (GRP) antiserum in the trigeminal ganglion (TG) and the caudal part of the medulla oblongata in male suncus. GRP-immunoreactivity was observed in a proportion of small neurons in the suncus TG (A) and in dense fiber projections to the caudal part of the medulla oblongata (B). Controls in which anti-GRP antiserum was preabsorbed with an excess of antigen peptide (50 μg/ml) showed a complete absence of GRP-expression in the TG (C) and caudal part of the medulla oblongata (D). Bars=50 μm (A, C); 500 μm (B, D).
The sensory modality transmitted by primary afferents is related to soma size distribution and associated axonal caliber and conduction velocity. Therefore, we compared the size distribution of TG neuron cell bodies between suncus and mice using fluorescent Nissl staining. The cell body areas of both male suncus and male mice showed a bell-shaped distribution up to about 1,000 μm2 with a peak at ~400 μm2 (Fig. 5). The expression of GRP as a marker for itch transmission was analyzed by combining IHC with Nissl staining. GRP-ir cells were mainly small- and medium-size TG neurons in both suncus (Fig. 5A) and mice (Fig. 5B). Subsequently, the proportion of the total and the size distribution of GRP-ir neurons in TG were analyzed. GRP-expressing TG neurons accounted for 8.6% of the total in suncus (549 out of 6,350 neurons measured from 4 males; Fig. 5C) and 6.6% in mice (498 out of 7,582 neurons in 4 males; Fig. 5D). There was no sex difference in the proportion of GRP-expressing TG neurons in suncus (7.9%, 984/12,479 from 4 females). Large neurons (>1,000 μm2) were rarely GRP-positive in either suncus or mice.

(A, B) Double fluorescence staining for Nissl substance (magenta) and GRP (green) in male suncus TG (A) and male mouse TG (B). Colocalization appears as white in the merged images (A, B). Histograms of GRP-positive and -negative soma sizes in suncus (C) and mice (D) TG neurons. GRP-immunoreactive neurons were predominantly small in the TG of both suncus and mice. Proportions of GRP-immunoreactive neurons: 8% of suncus TG neurons (C) and 6% of mouse TG neurons (D). Bar=100 μm.
We next examined the projection sites of GRP-ir neurons in the TG of suncus and mouse. GRP-ir fibers were sparse at the Vp level (Fig. 6A, B), Vo level (Fig. 6C, D), and Vi level (Fig. 6E, F) in both suncus and mice. In both suncus and mice, distinct GRP-ir fibers and terminals were observed in the transition area between Vi and Vc within the substantia gelatinosa, forming a sigmoid-like curve (Fig. 6G, H). At the Vc level, a large number of intense GRP-ir axons and terminals were detected in the superficial layers throughout the dorso-ventral and rostro-caudal extensions of the Vc (Fig. 6I, J). Strikingly, a dense accumulation of GRP-ir axons similar to those in the Vc was observed throughout the superficial layers of the cervical spinal dorsal horn in both suncus (Fig. 6K) and mice (Fig. 6L). Male and female suncus showed the same projection patterns of the GRP-ir fibers in the brainstem. Therefore, Figure 6 showed the male suncus brainstem and spinal cord as representative images.

Distribution of GRP in the trigeminal sensory nuclei and cervical spinal dorsal horn of male suncus and male mouse. GRP-ir fibers were sparse in the Vp (A, B), Vo (C, D), and Vi (E, F) in both suncus and mice. GRP-immunoreactivity was especially dense in the substantia gelatinosa, forming a sigmoid-like curve pattern in the transition area between Vi and Vc (G, H). GRP-positive fibers were also dense in the superficial laminae of the Vc in suncus (I) and mice (J). GRP-immunoreactivity was observed in the superficial laminae of the cervical spinal dorsal horn in suncus (K) and mice (L). Bars=500 μm.
We examined the expression of Grpr mRNA in the Vc and cervical spinal dorsal horn of suncus by RT-PCR. Total RNAs from the Vc and the cervical spinal dorsal horn of adult male and female suncus (n=3 of each sex) were reverse-transcribed. The resultant cDNA mixture was used for PCR amplification using specific primers for the Grpr gene. Bands were detected at the sizes expected for Grpr genes (545 bp) (Fig. 7A), indicating that Grpr mRNAs are expressed in the Vc and cervical spinal dorsal horn of male suncus. The Grpr mRNA band of female suncus was similar to that of males (data not shown). Negative controls in which the RT treatment was omitted showed a complete absence of bands in the Vc and cervical spinal dorsal horn (Fig. 7A).
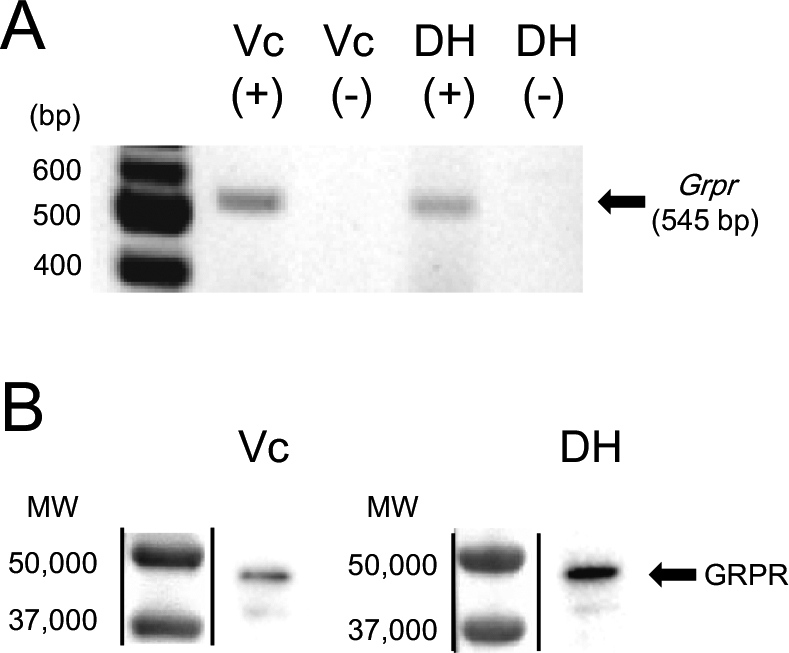
Expression of gastrin-releasing peptide receptor (GRPR) at the mRNA and protein levels in male suncus. Reverse transcription-PCR analysis (A) and Western immunoblot (B) were performed in the spinal trigeminal nucleus caudalis (Vc) and cervical spinal dorsal horn (DH) of suncus. A single band with the expected size (545 bp) of the amplicon was observed (A). The GRPR antiserum recognized a band at the expected molecular weight of GRPR (~43 kDa) on Western immunoblots of suncus Vc and DH (B). MW, molecular weight.
Western immunoblot analysis using anti-GRPR antiserum revealed a single band at approximately the expected molecular weight of GRPR (~43 kDa) in lysates prepared from the Vc and cervical spinal dorsal horn of both male suncus (Fig. 7B) and female suncus (data not shown).
We examined the comparative anatomy and expression patterns of GRP/GRPR in the trigeminal sensory system of a specialized placental mammal (suncus) and a laboratory animal (mouse). This study provides evidence for conservation of the trigeminal GRP/GRPR system mediating itch sensation across mammalian species.
Suncus is a small laboratory rodent slightly larger than mouse. Despite the relatively small body size of suncus, it has a disproportionately large TG and extended and flattered medulla oblongata compared to mice and rats [9]. We also found that suncus possesses an enlarged caudal spinal trigeminal nucleus, the area associated with perception of pain and itch sensation, suggesting that the trigeminal somatosensory system in suncus is well developed. Therefore, suncus may be a useful laboratory animal to study functional specialization of the trigeminal somatosensory system.
It is well accepted that small neurons in the spinal DRG and brainstem TG with C-fibers transmit itch, slow pain, and thermoception, whereas medium-size neurons with Aδ-fibers transmit fast pain and thermoception, and large neurons with Aβ-fibers transmit tactile and pressure sensations [5, 6, 18, 25, 32]. GRP-immunoreactivity was located mainly in smaller DRG neurons in mice [2, 8, 11, 12, 26, 34] and rats [28], and also smaller TG neurons in rats [28]. We found a similar distribution of GRP-ir neurons in suncus and mouse TG neurons. GRP-expressing neurons account for 2%–9% of mouse DRG neurons [2, 8, 11–13, 26, 34], 6% of rat DRG neurons [28], and 12% of rat TG neurons [28]. In this study, we also found that ~9% of TG neurons in suncus and ~7% of TG neurons in mice expressed GRP. Both the expression rate and localization of GRP in suncus and mouse TG neurons are generally consistent with a previous investigation in rats [28]. Some GRP-ir neurons co-expressed with calcitonin gene-related peptide and transient receptor potential vanilloid 1 in suncus TG (data not shown) as reported previously in rodents [1–3, 13, 26, 28, 34]. These results suggested that GRP neurons in the trigeminal somatosensory system of suncus and mice may participate in similar sensory functions. Accordingly, GRP may mediate the transmission of itch sensation in suncus. We found no sex difference in the proportion of GRP-ir TG neurons in suncus. Similarly, no sexual dimorphism between adult male and female rats was reported for GRP-ir intensity in the spinal dorsal horn [20, 21]. Taken together, these results suggest that the somatosensory GRP/GRPR system in eutherians is essential for both sexes.
We previously reported that GRP-ir fibers in rats specifically project to the Vc in the trigeminal sensory system and to the spinal dorsal horn [28]. In the present study, GRP-ir fibers were sparse in the Vp, Vo, and Vi, but dense in the Vc and spinal dorsal horn of both suncus and mice, consistent with our previous rat study [28]. Interestingly, GRP-ir intensity in TG neurons and GRP-ir fiber density in the Vc and cervical spinal dorsal horn were much higher in suncus than mice. Although we did not conduct quantitative analysis of GRP-ir intensity in these regions, this may reflect greater development in suncus than in mice. Such a result is consistent with their large TG and expanded caudal trigeminal nuclei of suncus, as an adaptation to a largely subterranean life.
GRPR mRNA and protein were expressed and localized in the Vc and cervical spinal dorsal horn as revealed by RT-PCR and Western blotting. The Vc and spinal dorsal horn are the dominant projection sites of GRP-ir primary afferents in rats [28] In this study, we showed a similar GRPR expression in mice, and suncus. These results indicate that itch-mediating GRP/GRPR synapses are localized in the Vc of the trigeminal somatosensory system and spinal dorsal horn of the spinal somatosensory system. These areas are considered essential relay points for itch transmission from the periphery. Further analyses focusing on GRPR-expressing neurons in the trigeminal somatosensory system are necessary because GRPRs are essential for the transmission of GRP signals to high brain centers.
The Asian house musk shrew Suncus murinus (order Eulipotyphla, formerly Insectivora) is believed to resemble specialized placental mammals. In Eulipotyphla, we demonstrate first time that the expression and distribution pattern of GRP in the trigeminal somatosensory system are similar to rodents [27, 28]. This anatomical insights suggests that the organization of the GRP/GRPR signaling system may be conserved in mammals. Itch sensation is perhaps universal among mammals, so the GRP/GRPR system may be involved in itch transmission in the trigeminal somatosensory system in suncus. Further studies on the somatosensory GRP systems of lower vertebrates such as birds and reptiles may provide new insight into the evolutionary origins and molecular mechanisms underlying itch sensation and its function. Although GRP and GRPR gene expression has already been reported in chicks [14, 16], its functional significance in the avian somatosensory system remains unclear. In addition, it is still unclear if this somatosensory GRP system exists and is functionally similarly in humans. Thus, future studies should include an emphasis on the comparative neurology of the GRP system in other vertebrates.
The authors declare no conflict of interest.
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Keiko T and HS. Acquisition of data: Keiko T, KI, and HM. Analysis and interpretation of data: Keiko T, KI, HM, Kei T, TJ, SO, MK, TS, and HS. Supply of experimental animals (suncus), advice, anatomy, and equipment: TJ and SO. Funding: Keiko T, MK, TS, and HS. Wrote the paper: Keiko T with assistance from HS. Study supervision: Keiko T.
The authors would like to thank Enago (www. enago.jp) for the English language review. This work was supported in part by KAKENHI from the Ministry of Education, Science, Sports, Culture and Technology (MEXT), Japan (to Keiko T; 26870496, 15J40220 and HS; 24680039, 15K15202, 15H05724), by the Ryobi Teien Memory Foundation, Japan (to HS). This work was also supported by MEXT KAKENHI (suncus genome resource; 221S0002). Keiko T is supported by Research Fellowships of JSPS for Young Scientists.