2017 Volume 50 Issue 1 Pages 49-55
2017 Volume 50 Issue 1 Pages 49-55
Mitochondrial ferritin (FtMt) is a novel iron storage protein with high homology to H-ferritin. Unlike the ubiquitously expressed H- and L-ferritin, FtMt is expressed in specific tissues such as the testis, heart, and brain. The function of FtMt is not fully understood; however, evidence suggests that it has a neuroprotective role in neurodegenerative diseases. We have previously reported that FtMt is expressed in catecholaminergic neurons of the monkey brainstem. To explore FtMt expression in human dopaminergic neurons, we designed a novel monoclonal antibody, C65-2, directed against human FtMt. Here, we report the properties of our C65-2 antibody. Western blots analysis and immunoabsorption tests demonstrated that the C65-2 antibody specifically recognized FtMt with no cross-reactivity to H-ferritin. Immunohistochemistry showed that the C65-2 antibody detected FtMt in neurons of the substantia nigra pars compacta (SNc) in humans and monkeys. We confirmed that FtMt is expressed in dopaminergic neurons of the human SNc. Our results suggest that FtMt is involved in various physiological and pathological mechanisms in human dopaminergic neurons, and the C65-2 monoclonal antibody promises to be a useful tool for determining the localization and biological functions of FtMt in the brain.
Mitochondrial ferritin (FtMt) is a recently-discovered ferritin encoded by an intronless gene on chromosome 5q23.1 [14]. FtMt is formed via a 242-amino acid precursor protein with a predicted molecular weight of 30 kDa. It is cleaved in the mitochondrial matrix to produce a mature protein approximately 22 kDa in size [15]. FtMt has 79% homology with H-chain ferritin (FTH). Both show ferroxidase activity and similar iron storage properties, but different properties have also been reported. FTH is localized in the cytoplasm, whereas FtMt is predominantly located in mitochondria. The FTH gene has iron-regulatory elements (IREs) and its expression is increased by iron [7, 9]. However, FtMt lacks IREs, and its expression is enhanced not by iron but by oxidative stress and inflammatory cytokines [14, 19]. FTH is ubiquitously expressed and at high levels in the liver and spleen, which contain high levels of iron. FtMt expression is not detected in the liver and spleen, but in other specific tissues such as the testis, heart, and brain [4]. This suggests that FtMt has functions that are not limited to H-ferritin-like roles; rather, it also has other functions in specific organs or under certain conditions.
In the brain, FtMt is expressed in neurons [16, 19] and is involved in neurological disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and restless legs syndrome (RLS) [16, 18, 19, 22]. Previous in vitro studies have reported that low FtMt expression is increased in neuroblastoma cells by oxidative stress [19, 21]. In addition, over-expression of FtMt has been found to protect neurons against oxidative stress [17, 19, 21, 23].
We previously generated a polyclonal anti-FtMt antibody and reported the distribution of FtMt in the monkey brainstem. FtMt expression was particularly observed in catecholaminergic neurons [24]. These results suggest that FtMt acts as a neuroprotective protein to maintain normal neuronal function. However, the functions of FtMt in the brain and its pathological significance in neurological disorders are still unclear. To address this, it is important to clarify the distribution pattern of FtMt in the human brain.
In the present study, we designed a monoclonal antibody (C65-2) against human FtMt. We demonstrated that C65-2 specifically recognized FtMt protein with no reactivity to FTH. This monoclonal antibody can be used for western blotting, immunohistochemistry and immunofluorescence analysis. Furthermore, its specificity makes it suitable for investigating the function of FtMt in human and monkey tissues. Using double immunostaining with the C65-2 antibody and a polyclonal antibody against tyrosine hydroxylase (TH), we confirmed the expression of FtMt in dopaminergic neurons in the substantia nigra pars compacta (SNc) of the human brain.
The current study protocols for animal use were assessed and approved by the Institutional Animal Care and Use Committee of Shiga University of Medical Science. For western blot analysis, the brainstem sample was obtained from a euthanized female cynomolgus monkey (age: 3 years and 10 months; weight: 2.67 kg). For immunohistochemistry, brains were obtained from two female cynomolgus monkeys (age: 5–11 years; weight: 3.38–4.68 kg). All efforts were made to minimize animal suffering and the number of animals used.
Human brainWe used postmortem human midbrain tissue from two individuals without neurological disorders (one 64-year-old male and one 72-year-old female). All procedures in this study were approved by the Ethical Committee of Shiga University of Medical Science (approval no. 28–26). We used human brain tissues in the brain bank of Shiga University of Medical Science. The postmortem human midbrains were fixed with formalin and then placed in 0.1 M phosphate buffer (pH 7.4) containing 15% sucrose and 0.1% sodium azide. The sucrose solution was changed repeatedly to remove all traces of formalin and to cryoprotect the tissue.
Tissue preparationTissue was prepared as previously described [1, 3]. Brain stem samples were removed from two cynomolgus monkeys after previous research use [1] and from human brains, as mentioned above. Brains were collected at different times and handled individually. Brains were fixed immediately with 4% formaldehyde in 0.1 M phosphate buffer (pH 7.4) for 3 days at 4°C. Next, the samples were immersed in 15% sucrose in 0.1 M PB with 0.1% sodium azide. The sucrose solution was changed daily for 4 days, after which the brains were stored in 15% sucrose solution at 4°C until processing. Samples were sectioned in a cryostat (Yamato, Japan) into 20-μm serial coronal sections that were floated in PBST (0.1 M phosphate buffered saline containing 0.3% Triton X-100, pH 7.4) and stored in PBST with sodium azide at 4°C.
Synthetic peptide design and generation of monoclonal antibodiesAntibodies against human FtMt were produced by Medical and Biological Laboratory Co. Ltd. (Ina, Nagano, Japan). Since FtMt has a high homology to H-ferritin, we selected TLGNENKQN in the C-terminal region as the immunizing peptide because this is specific to human FtMt (amino acid number 234–242, GenBank accession number BC034419). To conjugate the peptide to a carrier protein, cysteine (C) was added to the N-terminal end of the peptide, generating a 10-mer peptide (NH2–CTLGNENKQN–COOH). This was chemically linked to the carrier protein keyhole limpet hemocyanin (KLH). The KLH-conjugated FtMt peptide was injected into two C57BL6 mice. Lymph nodes were taken from the mice and processed for B cell hybridoma fusions. Hybridoma cloning was performed using ELISA methods to detect positive signals for the FtMt peptide. Several clones were obtained and one hybridoma, C65-2, was selected that displayed the highest titer for C65-2. Hybridoma cells were inoculated into the abdominal lumen of SCID mice and ascitic fluid was obtained. The monoclonal antibody was purified by ammonium sulfate precipitation and affinity chromatography using a protein A column. The purified antibody was stored in 10 mM phosphate buffered saline (PBS), pH 7.4, containing 0.1% sodium azide and 50% glycerin. Antibody specificity was assessed by western blot and immunoabsorption analysis as described below.
Western blot analysisWestern blot analysis was performed to confirm the specificity of the FtMt antibody. Brainstem tissue was homogenized in a glass homogenizer with RIPA buffer as described previously [2, 24] with slight modifications. Protein concentrations were assayed using a protein dye assay reagent (Bio-Rad Laboratories, Hercules, CA, USA). Approximately 5 μg of crude protein, prestained precision protein standards (Bio-Rad Laboratories), and Protein Ladder One Triple-color (NacalaiTesque, Kyoto, Japan) were electrophoresed on 15% or 5–20% SDS-polyacrylamide gels (Wako Pure Chemical Industries, Osaka, Japan) under reducing conditions and then transferred to polyvinylidenedifluoride membranes (Immobilon-P, Merck Millipore, Billerica, MA, USA). Membranes were incubated for 1 hr at room temperature (RT) with 20% Blocking Reagent-N102 (NOF Corp., Tokyo, Japan) in 25 mM tris-buffered saline containing 0.1% Tween-20 (TBST), at pH 7.4. After blocking, membranes were labeled for 8 hr with the monoclonal FtMt antibody (0.5 μg/mL) at 4°C. After three 10-min washes with TBST, the membranes were incubated for 1 hr in a peroxidase-labeled anti-mouse IgM (1:50,000; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at RT. Peroxidase labeling was detected by chemiluminescence using the Super Signal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA, USA).
To determine specificity of the staining, we performed an immunoabsorption test. The C65-2 antibody was pre-incubated with the immunizing FtMt peptide (1.0 μg/mL) for 24 hr at 4°C, and the membranes were incubated as described above.
ImmunohistochemistryImmunohistochemical staining for FtMt was performed as previously described [1, 24]. In brief, sections were kept at 4°C for at least 3 days in PBST before staining. Sections were incubated for 40 min in PBST containing 0.3% H2O2 and 0.1% sodium azide at RT. After three 10-min washes in PBST, sections were incubated for 1 hr with PBST containing 2% BSA. Next, the sections were incubated for 3 days with purified FtMt antibody (0.5 μg/mL) at 4°C. After washing, the sections were incubated for 1 hr in biotinylated anti-rabbit IgM (diluted 1:2,000; Vector Laboratories, Burlingame, CA, USA) at RT. Sections were finally incubated for 1 hr with an avidin–biotin–peroxidase complex (1:3,000 dilution; ABC Elite; Vector Laboratories) at RT, and peroxidase-labeled sections were developed in 0.02% 3,3-diamine-benzidine tetrahydrochloride with 0.3% nickel ammonium sulfate in 50 mM Tris–HCl (pH 7.6), with 0.005% hydrogen peroxide. PBST washes were performed between all steps.
Absorption testFor the immunoabsorption test, the purified antibody was pre-incubated with FtMt peptide at different concentrations (20 μg/ml, 5 μg/ml, 1.25 μg/ml, 0.3 μg/ml, and 0.075 μg/mL) for 1 hr at 4°C. Then the sections were stained as described above. No staining was observed following pre-incubation of the antibody with the immunizing FtMt peptide.
Immunofluorescence stainingThe co-localization of FtMt with TH-positive neurons in human midbrain sections was examined by double labeling with mouse monoclonal antibody against FtMt (C65-2) and rabbit polyclonal antibody against TH (Bioscensis Inc. Thebarton, Australia), followed by fluorescently labeled secondary antibodies. Some sections were incubated at 4°C overnight with a mixture of C65-2 and TH antibody (1:5,000). After washing, sections were incubated at RT for 2 hr in a mixture of Alexa Fluor 488-labeled chicken anti-rabbit IgG and Alexa Fluor 647-labeled donkey anti-mouse IgG secondary antibodies (1:500 dilutions; Molecular Probes Inc., Eugene, OR, USA). Sections were then washed three times for 10 min in PBS and mounted onto coated glass slides. Fluorescence was detected using a scanning laser confocal microscope (TE2000-E, Nikon, Tokyo, Japan).
MicrographsDigital images were obtained with a Nikon-D90 digital camera (Tokyo, Japan) adapted to an Olympus Microscope BX50 (Tokyo, Japan). Brightness and contrast were adjusted using Adobe Photoshop and no further image manipulation was performed.
The FtMt antibody, C65-2, was tested by western blot analysis and immunoabsorption. The total crude protein and isolated mitochondria and cytosolic fractions were extracted from monkey brainstem (Fig. 1). The C65-2 antibody detected a single band of approximately 22 kDa, corresponding to the molecular weight of FtMt in total crude protein (arrow in Fig. 1, lane 1). No bands were observed when the antibody was pre-absorbed with the FtMt peptide (Fig. 1, lane 2) or no primary antibody was used (Fig. 1, lane 3). The antibody detected FtMt in mitochondria fractions (Fig. 1, lane 4), but not in cytosolic fractions (Fig. 1, lane 5).
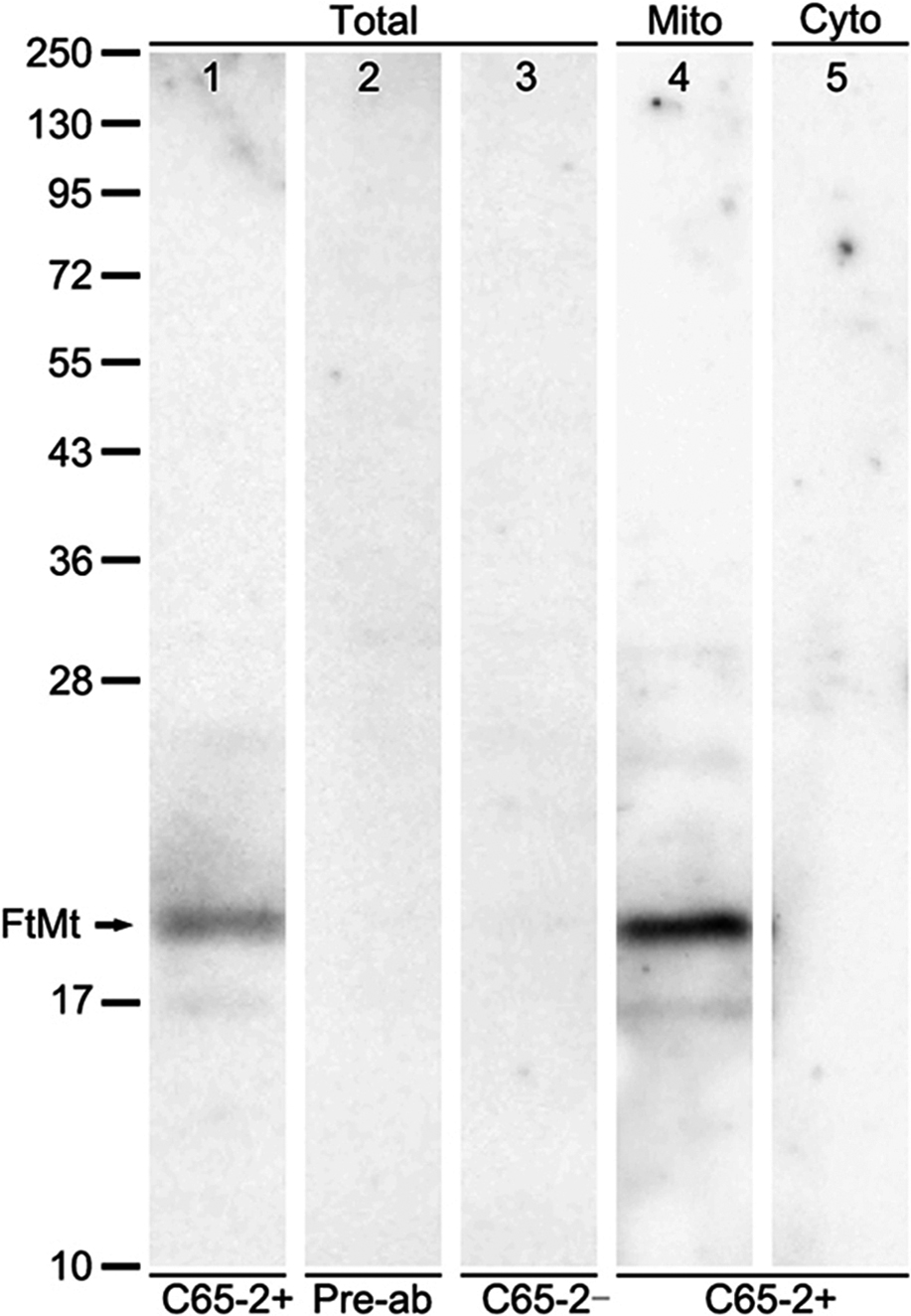
Western blot analysis in the monkey brainstem. Western blot analysis of total crude protein (Total), isolated mitochondria fraction (Mito), and cytosolic fraction (Cyto) extracted from a monkey brainstem. Adjacent lanes were loaded with equivalent amounts of protein. The FtMt monoclonal antibody (C65-2+) recognized a single band at approximately 22 kDa (lane 1). No bands were observed when the antibody was pre-absorbed with FtMt peptide (Pre-ab, lane 2) or absent (C65-2−, lane 3). The antibody detected FtMt in the mitochondrial fraction (lane 4), but not in the cytosolic fraction of the monkey brainstem (lane 5).
FtMt has a high homology with FTH; therefore, we confirmed that this monoclonal anti-FtMt antibody did not cross-react with FTH by western blot analysis (Fig. 2). FtMt and FTH-GFP fusion proteins were over-expressed in HEK293 cells and cell lysates were probed with anti-FtMt antibody (C65-2) and anti-GFP antibodies. C65-2 recognized the FtMt protein at a molecular weight of 22 kDa (Fig. 2, lane 1), but did not cross-react with FTH (Fig. 2, lane 2). The anti-GFP antibody did not recognize FtMt (Fig. 2, lane 3), but detected the FTH-GFP fusion protein (FTH, 21 kDa; GFP, 27 kDa) as a band with a molecular weight of 48 kDa (Fig. 2, lane 4).

Western blot analysis in HEK293 cells lysates. HEK293 cells were transfected with plasmids expressing FtMt (T-FtMt) or FTH-GFP (T-FTH-GFP). The transfectants extracted from HEK293 cells were probed with FtMt monoclonal antibody (C65-2) and GFP antibody (GFP). C65-2 recognized the FtMt protein around 22 kDa (lane 1), but did not detect FTH (lane 2). The GFP antibody did not detect FtMt (lane 3), but detected the FTH-GFP fusion protein (GFP, 27 kDa, FTH, 21 kDa) at about 48 kDa (lane 4). This shows that the FtMt monoclonal antibody does not cross-react with FTH.
In tissues, the C65-2 antibody stained some neuronal cell bodies in the SNc of cynomolgus monkey (Fig. 3) and human (Fig. 4) midbrains. Seen by high magnification, FtMt-immunoreactivity was localized to granule-like structures in neurons in both the monkey and human tissues (Fig. 3B and Fig. 4B). The staining intensity of FtMt-immunoreactivity was much decreased with the use of the antibody pre-incubated with 0.4 μg/ml of the FtMt peptide (Fig. 3C), and was abolished when the antibody was pre-absorbed with 1 μg/ml of the peptide (Fig. 3D).

FtMt immunohistochemistry and pre-absorption test in monkey tissues. A: Immunostaining of FtMt in the substantia nigra pars compacta of the monkey, using the C65-2 monoclonal anti-FtMt antibody. B: A high magnification of the boxed area B in Fig. 3A. FtMt-positive granules are seen in neuronal cell bodies. C and D: Immunoabsorption tests. The staining intensity of FtMt-immunoreactivity was much decreased with the use of the antibody pre-incubated with 0.4 μg/ml of the FtMt peptide (C). No positive staining was detected when the antibody was pre-absorbed with 1 μg/ml of the peptide (D). Bars = 50 μm.

FtMt-immunohistochemistry in the substantia nigra (SNc) and ventral tegmental area (VTA) of postmortem human brain. A: FtMt-positive neurons distributed in the SNc and VTA. A: FtMt-positive neurons are distributed in the SNC and VTA. B: A high magnification of the boxed area A in Fig. 4A. FtMt-positive granules are seen in both neuromelanin-positive (arrows) and -negative (arrowhead) neuronal cell bodies. C: A high magnification of the boxed area B in Fig. 4A. FtMt-positive granules are seen in neurons containing neuromelanin (arrow). A few neuromelanin-positive neurons do not contain FtMt-immunoreactivity (arrowhead). D: FtMt-immunostaining was abolished using G2-A pre-absorbed with 5 μg/ml of the FtMt peptide. Bars = 250 μm (A), 50 μm (B–D).
In the human midbrain, neurons in the SNc were strongly stained with C65-2 (Fig. 4A). The positive neurons were distributed from the SNc to the ventral tegmental area (Fig. 4A). At high magnification, FtMt-positive granules were seen in neuronal cell bodies containing neuromelanin (arrows in Fig. 4B and 4C). FtMt-positive granules were also observed in some neurons without neuromelanin (arrowhead in Fig. 4B). A few neurons containing neuromelanin do not contain FtMt-positive granules (arrowhead in Fig. 4C). The FtMt-positive granules were abolished with the use of C65-2 pre-absorbed with the peptide (Fig. 4D).
Localization of FtMt in catecholaminergic neurons in the human SNcTo investigate the expression of FtMt in catecholaminergic neurons of the human SNc, double immunofluorescence staining was performed. We labeled human brainstem samples with C65-2 (Fig. 5A) and anti-TH (Fig. 5B) antibodies. Our results showed considerable co-localization of FtMt and TH in neurons of the human SNc (Fig. 5C). A few neurons showed TH-positive and FtMt-negative (arrows in Fig. 5B and C). Co-localization occurred mainly in the neuronal cell bodies, consistent with our previous immunohistochemical observations using DAB (Fig. 4). A high percentage of neurons were doubly stained when compared with the total number of TH-positive neurons in the SNc (88.16%).

Double immunofluorescent staining for mitochondrial ferritin (A; FtMt, green) and tyrosine hydroxylase (B; TH, red) as well as the merged image (C). White arrows indicate TH-positive and FtMt-negative neurons. Bar = 50 μm.
In the current study, we produced a monoclonal antibody against an amino acid sequence specific to human FtMt. Antibody specificity was confirmed by western blot analysis and immunoabsorption. When we examined FtMt expression in the monkey brainstem by western blotting, C65-2 detected a single band with an approximate molecular weight of 22 kDa. This band was not observed when the FtMt antibody was omitted or pre-absorbed with antigenic peptide (CTLGNENKQN). In addition, western blot analysis confirmed that C65-2 did not cross-react with FTH, which shares 79% coding homology with FtMt in HEK293 cells. Immunohistochemistry using the antibody revealed FtMt-positive neurons in the SNc of monkey and human brainstems. This staining was abolished by pre-absorption with the FtMt peptide dose-dependently. These results indicate that C65-2 specifically recognizes FtMt, as seen by western blotting and immunohistochemistry.
FtMt in the catecholaminergic neurons of monkey and human SNcWe observed FtMt-positive neurons in the SNc of monkey and human midbrains. Double immunofluorescent staining for FtMt and TH indicated that 88.16% of TH-positive cells also expressed FtMt in the SNc of the human brainstem. This is consistent with our previous findings (69.18%) in the SNc of Macaca fascicularis [24]. Although the exact reasons for the difference remain unknown, they may be due to differences of iron metabolism involving neuromelanin.
Iron is an important transition metal that is stored in large amounts in the brain and tends to accumulate with aging [4, 26]. Previous studies have shown that iron is richly distributed in the substantia nigra of the normal adult human brain [13, 27], and that iron concentrations increase with age [25]. In the present study, monkey brainstem samples were obtained from young monkeys and the human midbrain samples were obtained from elderly individuals (62 and 72 years). Brown-colored neurons were observed in unstained human sections, indicating neuromelanin. Iron is involved in the synthesis of neuromelanin, which is found in the catecholaminergic neurons of the SNc and accumulates with age [25, 27]. In addition, considerable evidence suggests that excess iron increases the formation of toxic reactive oxygen species (ROS) [6, 20].
FtMt immunoreactivity was mainly observed in pigmented neurons of the SNc in the human midbrain. Previous studies have shown that the pigmented neurons contain neuromelanin and are more prone to degeneration and more sensitive to ROS than non-pigmented neurons in the SNc [10, 12]. These neurons may be preferentially targeted in PD [8, 11]. We previously demonstrated that mRNA and protein expression of FtMt are increased in vitro by pathological conditions such as accumulation of amyloid beta and ROS [5]. Interestingly, FtMt in midbrain dopaminergic neurons may be influenced by neurological disorders such as PD [17] and RLS [18]. This might explain the greater number of FtMt- and TH-double positive neurons in the SNc of the human brains compared with monkeys.
Our novel FtMt monoclonal antibody, C65-2, is suitable for the analysis of human tissue and may be useful for clarifying the functions of FtMt in dopaminergic neurons in physiological and pathological conditions.
In conclusion, we designed a novel human FtMt-specific monoclonal antibody and characterized it. The antibody did not cross react with FTH in the brain. We further explored the expression of FtMt in TH-positive dopaminergic neurons in the SNc of the human midbrain, and found that FtMt may be involved in aging in the human SNc. This study shows that our novel FtMt monoclonal antibody, C65-2, is suitable for the analysis of FtMt expression in the human brain.
All authors have no conflicts of interest to disclose.
We thank all members of the laboratory who helped with this work and provided suggestions for brain mapping. Dr. Mingchun Yang thanks the Otsuka Toshimi Scholarship foundation which provided a scholarship. The authors would like to thank Mr. T. Yamamoto of the Central Research Laboratory, Shiga University of Medical Science, for his excellent technical assistance with confocal microscopy.
This study was supported by Grant-in-Aids for Scientific Research (B) (Grant Number 26290022, I.T.) from the Japan Society for the Promotion of Science (JSPS).