2018 Volume 51 Issue 3 Pages 111-118
2018 Volume 51 Issue 3 Pages 111-118
The TRK-fused gene (TFG) is reported to be involved in the regulation of cell size, apoptosis, cell growth, ER-Golgi protein secretion, NF-κβ pathway signaling, the ubiquitin-proteasome system, and pancreatic β-cell mass and function. TFG mutations were reported in some neurodegenerative diseases affecting sensory and motor functions. However, the function of TFG in the nervous system and how TFG mutations lead to neurodegeneration remain unclear. In this study, we employed double immunohistochemistry to investigate the details of TFG localization patterns in monoaminergic and cholinergic neurons in the brainstem. Intense TFG immunoreactivity was observed in the dorsal raphe nucleus, the locus coeruleus, and the ventral horn of the spinal cord. TFG immunoreactivity was observed in some serotonergic neurons in all B1–B9 cell groups, and some noradrenergic neurons in all A1–A7 cell groups in the rat brainstem, while no immunoreactivity was observed in the dopaminergic neurons in A8–A10 cell groups. TFG immunoreactivity was observed in all ChAT-positive motor nuclei in the lower corticospinal tract of the rat brainstem.
The TRK-fused gene (TFG in human, Tfg in rat) was originally reported as a part of a chimeric oncogene fused with the growth factor receptor, NTRK1 (TRK-A) in human papillary thyroid carcinoma [6, 9]; later TFG gene was identified to be expressed in various normal tissues including the nervous system [19]. TFG mutations were found in patients affected with different neurological diseases, including motor and sensory functions in hereditary motor and sensory neuropathy with proximal dominant involvement (HMSNP) [10, 13], complicated hereditary spastic paraplegias [1] and Charcot-Marie-Tooth disease type II (CMT2) [30].
We have previously reported that a Tfg spliced variant product having an alternative exon 7b and lacking exon 8 was expressed in the rat nervous system concomitantly with conventional Tfg [15]. Furthermore, it has been reported a similar TFG spliced variant is expressed in the human nervous system [10]. However, the role for TFG in the nervous system is still unresolved. Studies on TFG showed that it is involved in regulation of cell size and apoptosis [2], cell growth [5], ER-golgi COPII-coated vesicle transportation [11, 35], the NFκβ pathway [5, 20], inhibitory function in ubiquitin-proteasome system [36], and pancreatic β-cell mass and function [37]. The TFG protein sequence in human, mouse, pig, Schistosoma mansomini show high homology [18], implying non-human model TFG studies can be useful for understanding TFG roles in human neurological disorders.
We previously produced an antibody against rat TFG, and reported that TFG is localized to neurons in specific regions in the rat brainstem such as the raphe nuclei, the gigantocellular reticular nucleus, the reticulotegmental nucleus of the pons, and some cranial nerve nuclei such as the trigeminal nuclei, the vestibulocochlear nuclei, and the dorsal motor nucleus of the vagus [15, 28]. Those neurons positive for TFG were partly colocalized to serotonergic neurons in rat brainstem [28].
In this study, we employed double immunohistochemistry to investigate colocalization patterns of TFG in serotonergic, noradrenergic and dopaminergic neurons in the brainstem areas positive for TFG immunoreactivity. We also investigated TFG immunoreactivity in choline acetyltransferase (ChAT)-positive motor neurons of the motor cranial nuclei and the ventral horn of the spinal cord to gain better understanding of TFG expression in the motor system.
Six male and 2 female Wistar rats weighing 220–400 g at 11 weeks of age were used in this study. The rats were purchased from Clea Japan (Osaka, Japan). All animal experiments were performed respecting the dignity of animal lives with protocols approved by the Institutional Animal Care and Use Committee of Shiga University of Medical Science. All animals were housed under a 12 hr:12 hr light-dark schedule. Food and water were given ad libitum. Animals were anesthetized with pentobarbital (80 mg/kg), and were transcardially perfused with 10 mM phosphate-buffered saline (PBS) followed by ice-cold 0.1 M phosphate buffer (PB; pH 7.4) containing 4% formaldehyde (FA). The brain and spinal cords were removed and postfixed for 24 hr at 4°C in 0.1 M PB containing 4% FA. The tissues were then immersed for at least 48 hr in 0.1 M PB containing 15% sucrose and 0.1% sodium azide for cryoprotection. The tissues were cut into 20 μm thick sections using a cryostat, and the sections were used in a free-floating state.
Antibody characterizationWe generated a rabbit polyclonal anti-TFG antibody using a synthetic peptide corresponding to the common region of TFG protein and its variant as antigen (SGPPSAPTEDRSGTP: amino acid number 194–208, Accession number BC078947 on GenBank), as previously reported [15]. The specificity of the antibody was assessed by western blot analysis and it recognized 30 kDa and 50 kDa bands in rat brain homogenate, corresponding to the molecular sizes of the conventional and variant forms of TFG [15]. As an immunohistochemical control, the antibody was incubated with the TFG peptide (100 μg/mL) with absorption resulting in no specific staining on the rat brain sections [28].
The following antibodies were used to identify monoaminergic and cholinergic neurons in this study. Goat polyclonal anti-ChAT antibody (Millipore, Cat#AB144P, RRID: AB_2079751, diluted 1:1000) to stain cholinergic neurons; mouse monoclonal anti-serotonin antibody was used to stain serotonergic neurons (29, diluted 1:1000); mouse monoclonal anti-dopamine beta hydroxylase (DBH) antibody (Millipore, MAB308, RRID: AB_2245740, diluted 1:1000) was used to stain noradrenergic neurons; and mouse monoclonal anti-tyrosine hydroxylase (TH) antibody (Millipore, MAB318, RRID: AB_2201528, diluted 1:1000) was used to stain dopaminergic neurons.
The ChAT antibody was raised against human placental enzyme, and absorption of ChAT antibody with ChAT protein resulted in no specific staining [14]. This antibody stained cholinergic neurons in the rat brainstem and in the ventral horn of the spinal cord. The serotonin antibody was raised against serotonin and its immunological property was assessed by an enzyme-linked immunoassay. Immunohistochemical controls using the preimmune serum and the antibody absorbed with serotonin showed no specific staining [29]. The DBH antibody was raised against purified bovine DBH. The antibody only stained cells known to express DBH in the rat brainstem sections, such as cells in the locus coeruleus, and cells in other known noradrenaline cell groups. The TH antibody was raised against tyrosine hydroxylase purified from PC12 cells. The antibody stained cells known to express TH in the rat brainstem, such as substantia nigra neurons. 0.1 M phosphate buffer (pH 7.4) containing 0.9% NaCl and 0.3% Triton X-100 (PBST) containing 0.2% bovine serum albumin (BSA) was used to dilute the antibodies.
Double fluorescence immunohistochemistryThe sections were immersed in MilliQ water for 1 min. The sections were incubated with a mixture of primary antibodies for 3 days at 4°C. After washing with PBST, the sections were incubated for 1 hr with a mixture of corresponding secondary antibodies conjugated with Alexa 488 or 546 and Alexa 647 (LifeTechnologies; chicken anti-rabbit, cat. no. A-21441 diluted 1:500; donkey anti-rabbit, cat. no. A-11040, diluted 1:500; donkey anti-mouse, cat. no. A-31571, diluted 1:500; chicken anti-goat, cat. no. A-21469, diluted 1:500) at room temperature. After mounting with Shandon Immu-Mount (Thermo Scientific, Cheshire, WA) on glass slides (Platinum Coat, Matsunami Glass, Osaka, Japan), sections were examined using a confocal microscope (Olympus, IX-81 FV1000-D, Tokyo, Japan). PBST containing 0.2% BSA was used to dilute the antibodies and PBST was used to wash the sections between each step. Contrast and brightness levels of all digital images were adjusted with Adobe Photoshop to the same levels.
To investigate TFG immunoreactivity in the rat monoaminergic neurons, we used an anti-serotonin antibody to stain serotonergic neurons, an anti-DBH antibody to stain noradrenergic neurons, and an anti-TH antibody to stain dopaminergic neurons, then performed double fluorescent immunohistochemistry with our anti-TFG antibody.
Figure 1 shows schematic drawings indicating localizations of serotonin cell groups B1–B9 (Fig. 1A), noradrenaline cell groups A1–A7 (Fig. 1B), and dopamine cell groups A8–A9 (Fig. 1C) in the rat brainstem based on Paxinos and Watson [25], Paxinos [24], and McGeer [17].

Schematic drawings of rat brainstem to illustrate the distribution of serotonergic (A), noradrenergic (B) and dopaminergic (C) neurons. Spots indicate the places where monoaminergic neurons are present. Corresponding levels: from Bregma −6.84 (1), from Bregma −7.80 (2), from Bregma −9.24 (3), from Bregma −10.80 (4), from Bregma −12.24 (5), from Bregma −12.84 (6), from Bregma −8.28 (7), from Bregma −9.72 (8), from Bregma −12.24 (9), from Bregma −14.40 (10), from Bregma −15.48 (11), from Bregma −6.24 (12), from Bregma −7.56 (13).
We observed TFG-positive neurons in all of the serotonin cell groups B1 to B9 in the rat brainstem (Fig. 2). TFG immunoreactivity was particularly prominent in all raphe nuclei; raphe pallidus nucleus (B1 cell group, Fig. 2A), raphe obscurus nucleus (B2 cell group, Fig. 2C), raphe magnus nucleus (B3 cell group, Fig. 2D), median raphe nucleus (B5 cell group, Fig. 2H), dorsal raphe nucleus of pons (B6 cell group, Fig. 2I), dorsal raphe nucleus of midbrain (B7 cell cell group, Fig. 2J), and median raphe nucleus (B8 cell group, Fig. 2K). TFG- and serotonin-positive neurons were observed in all serotonin cell groups in the rat brainstem, but the intensity of TFG immunoreactivity was different in each group. TFG immunoreactivity detected in serotonergic neurons in central gray (B1 cell group, Fig. 2B) and medial lemniscus (B9 cell group, Fig. 2L) was much less than that observed in the raphe nuclei. TFG immunoreactivity was also detected in all noradrenaline cell groups in the rat brainstem (Fig. 3). In locus coeruleus, TFG-positive neurons were detected in many population of neurons with dense immunoreactivity (A6 cell group, Fig. 3E). Beside the A6 cell group neurons in the locus coeruleus, TFG immunoreactivity was observed in other noradrenaline cell groups. TFG immunoreactivity was limited in areas containing dopaminergic neurons in the rat brainstem. TFG immunoreactivity was not detected in dopaminergic cells positive for an anti-TH antibody of rat brainstem (Fig. 4).

Double immunostaining for TFG (green), serotonin (red), and merged images in the serotonin cell groups. B1 cell group in raphe pallidus nucleus (RPa) (A), B1 cell group in caudoventrolateral reticular nucleus (CVL) (B), B2 cell group in raphe obscurus nucleus (ROb) (C), B3 cell group in raphe magnus nucleus (RMg) (D), B3 cell group in lateral paragigantocellular nucleus (LPGi) (E), B3 cell group in rostroventrolateral reticular nucleus (RVL) (F), B4 cell group in central gray of medulla (CG) (G), B5 cell group in median raphe nucleus (MnR) (H), B6 cell group in dorsal raphe nucleus of pons (pons DR) (I), B7 cell group in dorsal raphe nucleus of midbrain (midbrain DR) (J), B8 cell group in median raphe nucleus of midbrain (Midbrain MnR) (K), B9 cell group in medial leminiscus (ml) (L). Arrowheads indicate merged neurons. Bar = 50 μm.
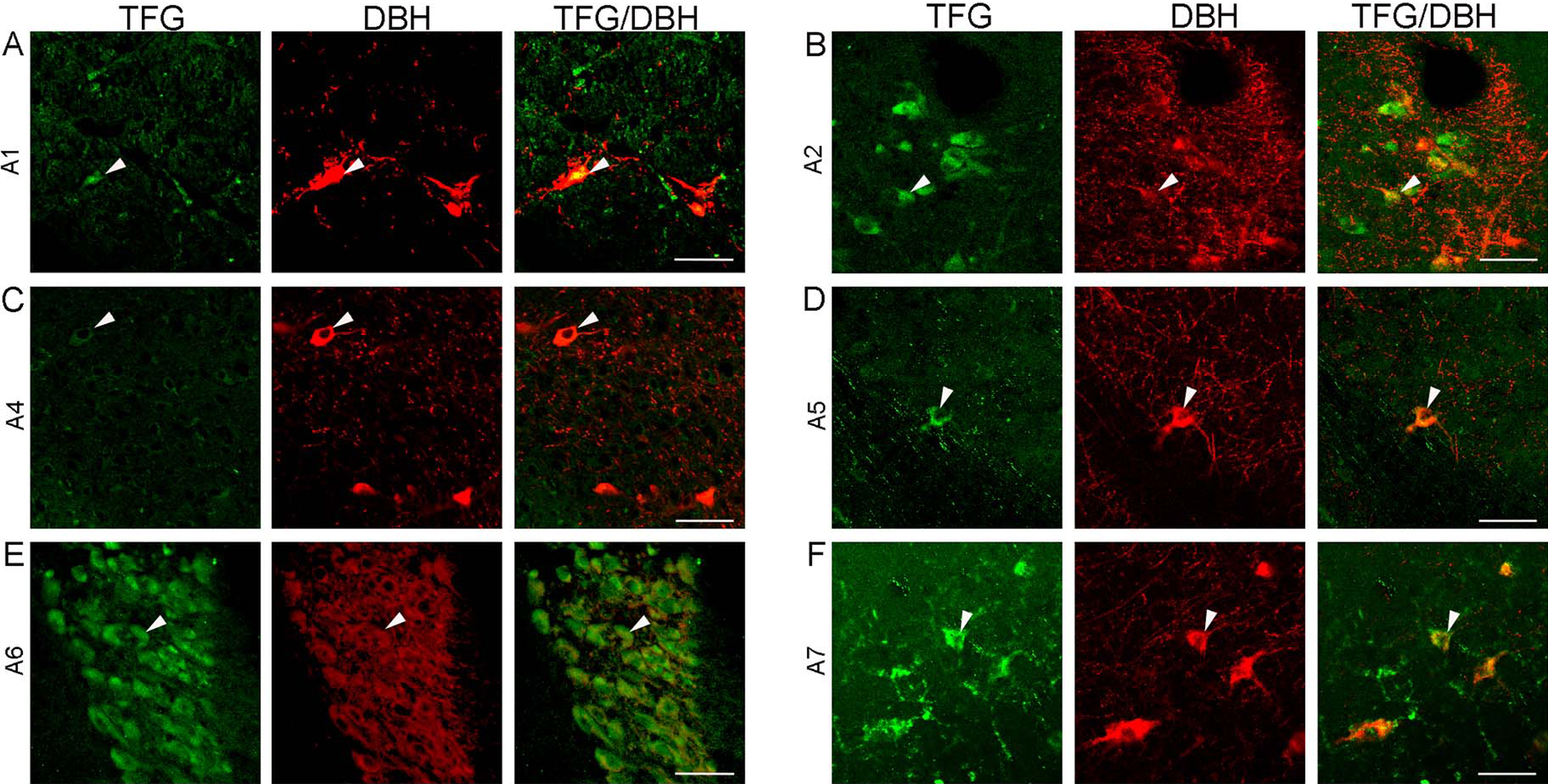
Double immunostaining for TFG (green), DBH (red), and merged images in the noradrenaline cell groups. A1 cell group (A), A2 cell group (B), A4 cell group (C), A5 cell group (D), A6 cell group (E), A7 cell group (F). Arrowheads indicate merged neurons. Bar = 50 μm.
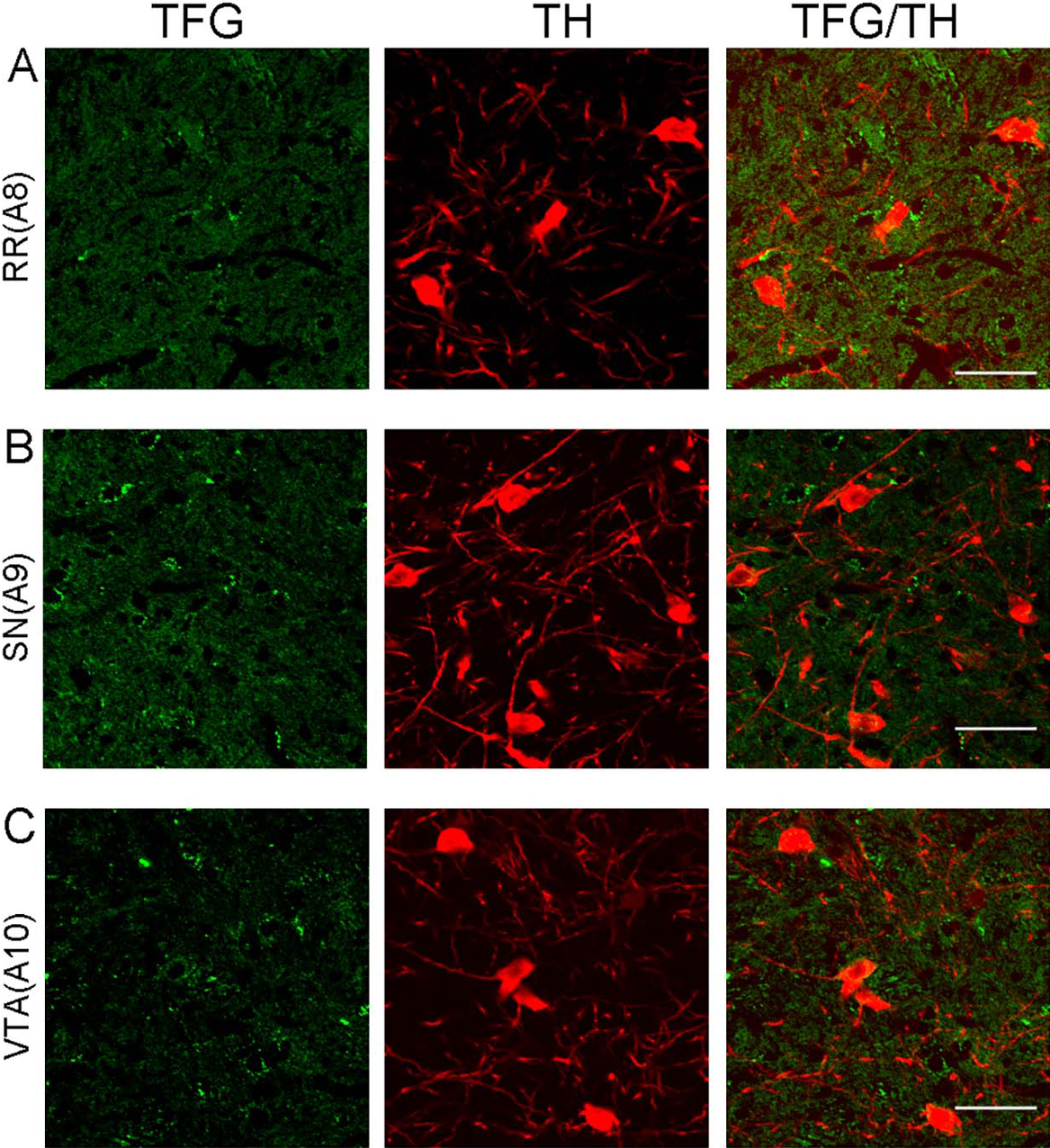
Double immunostaining for TFG (green), TH (red), and merged images in the dopamine cell groups. A8 cell group in retororubral nucleus (RR) (A), A9 cell group in substantia nigra (SN) (B), A10 cell group ventral tegmental area (VTA) (C). Bar = 50 μm.
We used anti-ChAT and anti-TFG antibodies to identify cholinergic motor neurons in the ChAT-positive motor neurons in the brainstem and the upper cervical spinal cord that were TFG positive. We assessed TFG immunoreactivity in the pedunculopontine tegmental nucleus (Ch5), the laterodorsal tegmental nucleus (Ch6), some cranial nuclei (oculomotor nucleus, trochlear nucleus, trigeminal nucleus, abducent nucleus, facial nucleus, dorsal motor nucleus of vagus, hypoglossal nucleus, and nucleus ambiguus) and ventral horn of the spinal cord. TFG immunoreactivity was observed in some neurons in the motor nuclei and ventral horn of spinal cord (Fig. 5), but not in the pedunculopontine tegmental nucleus (Ch5) or the laterodorsal tegmental nucleus (Ch6) (data not shown). TFG staining was present in most ChAT-positive neurons in the ventral horn of the upper cervical spinal cord (Fig. 5E). Additionally, we investigated TFG immunoreactivity in GABAergic neuron using the antibody against Glutamate decarboxylase, but we could not detect TFG immunoreactivity in neurons stained for GABAergic neurons in the rat brainstem (data not shown).

Double immunostaining for TFG (green), ChAT (red), and merged images in the corticospinal tract lower motor neurons. Trigeminal motor nucleus (A), facial motor nucleus (B), hypoglossal nucleus (C), nucleus ambiguus (D), ventral horn of the spinal cord (E). Arrowheads indicate merged neurons. Bar = 200 μm.
Previously, Ishiura et al. investigated TFG immunoreactivity in the brain and spinal cord of a TFG-mutation patient, and detected TFG immunoreactivity in the motor neurons of the facial, hypoglossal, and abducens nuclei, and the spinal cord, as well as in the sensory neurons of the dorsal root ganglia [10]. We observed TFG immunoreactivity in the motor neurons of the facial, hypoglossal, and abducens nuclei and the spinal cord neurons in the normal rat brainstem, but we did not investigate TFG immunoreactivity in the sensory neurons of the dorsal root ganglia. Our findings are in agreement with the findings by Ishiura et al.
In rat, TFG immunoreactive neurons were detected in all of the serotonin cell groups B1–B9 areas; particularly strong immunoreactivity was observed in the dorsal raphe nucleus. About 28 percent of TFG immunoreactive neurons in the dorsal raphe nucleus colocalized with serotonin-positive neurons as reported previously [28]. In serotonin cell groups, TFG immunoreactivity was detected prominently in midbrain dorsal raphe nucleus and midbrain median raphe nucleus, both of which are derived from anterior rhombomeres of the embryonic hindbrain [23]. The serotonergic neurons in the raphe nuclei have been suggested to be more heterogeneous with their cellular and genetic phenotypes [23], and it is possible that TFG-positive serotonergic neurons have a specific physiological role. However, we detected TFG immunoreactivity throughout all the serotonin cell groups in the rat brainstem and also in serotonin-negative neurons. We can not suggest a specific serotonin subpopulation type in those TFG and serotonin positive neurons in this study. Half of the serotonergic neurons in the serotonin cell groups are contained in the dorsal raphe nucleus [4, 34]; however, the dorsal raphe nucleus contains other neurotransmitters besides serotonin, such as noradrenaline [7] and dopamine [21]. Noradrenergic neurons are found in cell group A1, A2, A4, A5, A6 and A7, and dopaminergic neurons are found in cell group A8–A10 of the rat brainstem [3]. To examine TFG immunoreactive neuronal types in noradrenergic and dopaminergic neurons in the rat brainstem, we performed double immunofluorescence using antibodies against DBH and TH, as noradrenergic and dopaminergic neuron marker respectively, combined with our TFG antibody. We detected TFG immunoreactive neurons in all A1, A2, A4, A5, A6 and A7 areas and some were DBH-positive, but we could hardly detect any TFG immunoreactivity in areas A8–A10 and no TFG immunoreactivity was detected in dopaminergic neurons. Many TFG immunoreactive neurons were observed in the locus coeruleus, where the A6 cell group is found. However, the locus coeruleus in the rat contains approximately 1500 neurons on each hemisphere [27]; this likely contributed to the dense appearance of TFG immunoreactivity in A6 cell group. Westlund et al. showed noradrenergic neurons projecting to the spinal cord are located in the A5–A7 cell groups [33].
The brainstem also contains Ch5 and Ch6 cholinergic neurons positive for ChAT antibody. Ch5 and Ch6 neurons are cholinergic cell groups that innervate the thalamus and the striatum. Ch5 neurons appear to be involved in processing for sensory system and extrapyramidal motor control, whereas the Ch6 neurons are involved in the limbic system [32]. We did not observe any TFG immunoreactivity in the ChAT-positive neurons in the pedunculopontine tegmental nucleus (Ch5) and the laterodorsal tegmental nucleus (Ch6).
In this study, TFG immunoreactivity was observed in some motor neurons in motor nuclei of the rat lower corticospinal tract. Motor neurons in some cranial nuclei and ventral horn of the spinal cord are part of the lower corticospinal tract.
TFG immunoreactivity was observed mainly in the neuronal cell bodies, varicose fibers and proximal dendrites in the TFG immunoreactive neurons [28]. TFG has been demonstrated to be involved in the formation of COPII-coated vesicles that transport from the endoplasmic reticulum to the Golgi, supporting secretary pathway organization [11, 35]. Immunohistochemistry using antibodies against serotonin and DBH shows immunoreactivities in the cell bodies and varicose fibers [16, 26], and we found TFG staining in the proximal processes and varicose fibers in some neurons, but the staining patterns for TFG are slightly different being often more condensed in the cell bodies. TFG immunoreactivities were detected in the same neurons positive for serotonin or DBH immunoreactivity, but the somata areas stained for TFG antibodies appeared often less than the somata areas stained for serotonin or DBH antibodies. This is probably due to TFG playing a role as a scaffold protein for COPII-vesicle protein at the ER-Golgi site, whereas serotonin and DBH are mainly found inside vesicles that localize more externally than TFG in the neurons.
Although the function of TFG in the nervous system remains unclear, mutations in TFG have been reported to be associated with rare neurological disorders. A heterozygous mutation (c.854C>T) in TFG has been reported to cause HMSN-P [10, 13], a different heterozygous mutation (c.806G>T) has been reported to cause CMT2 [30], and the homozygous (c.316C>T) mutation has been reported to cause complicated hereditary spastic paraplegias [1]. All reported disorders share common features such as motor impairment, sensory neuropathy and axon degeneration, although the neurological manifestations varied among the patients.
The serotonin system extensively affects various areas of brain, and is implicated in sleep/arousal, learning/memory, sensory perception, motor coordination, pain regulation, and ingestive behaviors [31]. TFG in serotonergic neurons may play a role in sensory systems, and its mutations may lead to sensory neuropathy reported in patients with TFG mutations.
Cholinergic neurons are involved in the pathogenesis of neurological diseases including Alzheimer’s disease, Huntington disease, Schizophrenia, Sudden infant death syndrome and Amyotrophic lateral sclerosis (ALS) [22]. ALS is a progressive motor neuron degenerating disease affecting spinal cord neurons and its pathogenesis is still unknown. Studies have shown lower ChAT activity in the spinal cord of ALS patients and myelin degeneration [8, 12]. HSMN-P and complicated hereditary spastic paraplegia with TFG mutations have similarities to ALS pathology, such as myelin degeneration and motor dysfunction [1, 13].
The TFG immunoreactivity pattern implies TFG function is associated with the pyramidal system. TFG mutations may affect cholinergic and noradrenergic neurons in the pyramidal system to manifest dysfunctions in motor system, since patients with TFG mutations have reported motor impairments [1, 13].
Our findings provide a profile of TFG expression in the monoaminergic and cholinergic neurons in the rat brainstem and the spinal cord. Further investigation of TFG and its associated proteins in disease models will be needed to reveal a role for TFG in the pathology of the neurodegenerative diseases with TFG mutations.
The authors declare no conflicts of interest.
We are greatly thankful to Dr. J.P. Bellier and Dr. D. Yanagisawa for their kind technical advice, Mr. T. Yamamoto of the Central Research Laboratory at Shiga University of Medical Science for his assistance in confocal microscopy, Prof. D. G. Walker for proofreading the manuscript for English, and Mr. T. Kohno and Mr. J. Munakata for their assistance in immunohistochemistry.