2019 Volume 52 Issue 4 Pages 77-83
2019 Volume 52 Issue 4 Pages 77-83
The interleukin (IL)-4, 1,25(OH)2D3 and retinoic acid, increase surface expression of functional integrin αvβ3 on murine osteoclast precursors. All three agonists stimulate transcription of the β3 gene, leading to increased steady-state levels of mRNA this protein. By contrast, mRNA levels of αv remain unchanged. In each instance, the increase in the surface expression of the integrin results in increased migration of the cells onto an αvβ3 substrate. Because β3 subunit, except platelet where β3 subunit conform a dimer with αIIb, associates solely with αv subunit monogamously, while promiscuous αv subunit combines with various subunit, our present data support the idea that the β3 subunit governs the surface-expressed functional integrin complex.
Integrins are transmembrane heterodimers comprising individual α and β subunits [11, 28]. Many of these complexes recognize and anchor cells to extracellular matrices, events associated with transmission of signals across the plasma membrane [14, 26]. Among various types of integrins, αvβ3 plays a central role in bone cell biology [26, 28], most importantly in terms of osteoclast function [26]. In the avian system, the osteoclastogenic 1,25(OH)2D3 and retinoic acid, have been shown to regulate the expression of αvβ3 by the activation of the β3 integrin gene [6, 21]. The same osteoclastogenic 1,25(OH)2D3 and retinoic acid are also known to modulate mammalian bone metabolism, which, at least in part, is achieved by controlling the differentiation of osteoclasts [7, 10, 16, 25]. We have previously reported that the hematopoietic cytokine, interleukin (IL)-4, increases integrin β3 subunit expression in murine osteoclast precursors by transactivating the gene [17]. To date, however, still not determined is whether 1,25(OH)2D3 and retinoic acid also contribute to modulating the expression of the heterodimer in murine osteoclast precursors.
In this study, we demonstrate the expression of the β3 integrin subunit in 1) bone marrow macrophages (BMMs) cultured with 1,25(OH)2D3, retinoic acid and interleukin (IL)-4, and 2) decalcified mouse tibiae from normal and IL-4 transgenic mice.
Bone marrow cells were obtained from 6-week-old male A/J mice (Jackson Lab, Benton Harbor, MI) by flushing the femora and tibiae with α-modified Eagle’s medium (α-MEM). Mouse macrophage colony stimulating factor (M-CSF) was, by the batch calcium phosphate method, purified to Stage 1 from the serum-free medium conditioned by mouse fibroblastic cell line L929 [9]. The cells were cultured in α-MEM supplemented with 10% charcoalstripped serum fetal bovine serum (FBS) in the presence of 500 U/ml of M-CSF for 24 hr. The non-adherent population was harvested and incubated in pronase solution [0.02% w/v pronase, 1.5 mM of EDTA in phosphate buffered saline (PBS)] for 15 min at 37°C. The suspension was layered on horse serum and incubated for 10 min at 4°C. The BMM-enriched population was recovered from the upper layer by centrifugation (1500 rpm for 5 min) and plated at 1.5 × 106 cells/ml in α-MEM with 10% charcoalstripped FBS and 500 U/ml of M-CSF for 2 days which yields, a pure population of M-CSF-dependent macrophage precursors, as described [11]. The adherent population of BMMs was then treated with a range of concentrations of 1,25(OH)2D3 and retinoic acid or with the vehicle for up to 3 days in the presence of 500 U/ml of M-CSF to obtain an optimal biologically effective concentration of 1,25(OH)2D3 and retinoic acid. In combination studies, IL-4 (purchased from Genzyme (Cambridge, MA)), a known modulator of murine β3 gene expression, was added at a biologically effective concentration (100 U/ml).
Northern blot hybridizationA full-length murine integrin β3 subunit cDNA [20] was 32P-dCTP labeled by the random priming method (Boehringer Mannheim, Indianapolis, IN). A partial chicken integrin αv subunit cDNA probe [2] was a kind gift from Dr. B. Bossy, University of California, San Francisco, CA. Total cellular BMM RNA was isolated by single-step acid guanidinium thiocyanate-phenol-chloroform (AGPC) extraction. Equal amounts of RNA (10 μg per lane) were electrophoresed in 1.0% agarose gels and transferred to nylon membranes with a vacuum blotter. The membranes were prehybridized with hybridization buffer [5 × SSPE, 5 × Denhardt’s solution, 50% formamide, 0.1% sodium dodecyl sulfate (SDS), 1 × Background Quencher, Tel-Test, Inc., Friendswood, TX] for 2 hr at 42°C, hybridized in hybridization buffer with 32P-labeled probe for 16 hr at 42°C, then washed with 2 × SSC-0.1% SDS for 30 min at 42°C and with 0.2 × SSC-0.1% SDS for 30 min at 60°C and exposed to Kodak X-OMAT film for 2 days at −80°C. The membranes were finally rehybridized with αv cDNA probe after stripping off the β3 blot. Optical density analysis (NIH ImageJ) was used to determine the relative abundance of 28S ribosomal RNA in each sample.
ImmunoprecipitationThe treated BMMs were rinsed free of the culture medium with PBS and labeled with 125I-lactoperoxidase for 1 hr at 27°C to screen total amount of β3 integrin subunit on the cell surface. The cells were then lysed in a minimal volume of lysis buffer [10 mM of Tris, pH 7.2, 150 mM of NaCl, 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS, 0.025% NaN3, 5 mM iodoacetamide, 1 mM of CaCl2, 1 mM of MgCl2, 4 mM phenylmethylsulfonyl fluoride (PMSF) and aprotinin 0.25 U/ml]. Each lysate, containing equal TCA-precipitable counts, was precleared with protein-G Sepharose, (Pharmacia Biotech, Inc., Piscataway, NJ), and immunoprecipitated with the polyclonal rabbit anti-β3 integrin subunit antibody raised against synthetic peptides corresponding to the cytoplasmic portion of the β3 integrin amino acid sequence (kindly donated by Dr. L. F. Reichardt [3] (Howard, Hughes Medical Institute, University of California San Francisco, CA). Excess amounts of antibody (10 mg/ml), determined by our previous immunoprecipitation study [17], was then added in order to quantitate the amount of αvβ3 expressed on the cell surface. The precipitate, recovered with excess protein-G Sepharose, was boiled in electrophoresis sample buffer and subjected to 6% SDS-polyacrylamide gel electrophoresis (PAGE) under reducing conditions. The gels were dried and exposed to Kodak X-OMAT film for 2 days at −80°C. Alternatively, for detection of non-covalently bound and surface-expressed integrin dimers [15], cells were washed in PBS-0.1M HEPES, and cell surface proteins were biotinylated using n-hydroxysuccinimide-biotin (NHS-biotin, Pierce Chemical Co., Rockford, Illinois, USA) and the manufacturer’s recommended conditions (450 μg NHS-biotin in 2.5 ml PBS-0.1 M HEPES per 150 mm2 plate, and then incubated for 45 minutes at room temperature). Cells were washed gently three times with cold PBS and then lysed in lysis buffer (10 mM Tris, 150 mM NaCl, 1.0 mM CaCl, 0.02% NaN3 2× protease inhibitor cocktail, and 2% Renex (Sigma Chemical Co.). Protein concentrations in the lysates were determined by bicinchoninic acid reaction (Pierce Chemical Co.), and equal amounts of total protein were immunoprecipitated. Optical density analysis (NIH ImageJ) was used to determine the relative abundance of protein in each sample.
Nuclear run-on assayRun-on assays were conducted in the presence of 32P-UTP, with nuclei from cells treated with the vehicle or with combinations of IL-4, 1,25(OH)2D3 or with only retinoic acid. Total RNA was isolated and hybridized to a membrane preslotted with excess β3, G3PDH or vector DNA. After stringent washing, the membrane was exposed to film as described [17].
In situ hybridizationA 500 bp murine β3 cDNA containing exons 3 and 4 [20] was subcloned into pGEM-3Z (Promega) and digoxygenin-labeled sense and anti-sense cRNA were transcribed, in vitro, with the use of the Riboprobe Gemini II transcription system (Boehringer Mannheim) and digoxygenin-UTP (Boehringer Mannheim) and used as hybridization probes. The treated BMMs cultured in chamber slides (Nunc, Naperville, IL) were washed 3 times with 100 mM phosphate buffer (PB, pH 7.4), and fixed with 4% paraformaldehyde (PFA) in 0.1 M of PB for 30 min at 4°C. Tibial bone removed from IL-4 transgenic [27] (kindly provided by Dr Philip Leder, Department of Genetics, Harvard Medical School) and normal mice was fixed with 4% PFA in 0.1 M of PB for 48 hr at 4°C, and decalcified with 20% EDTA for 4 days. Sections were cut from paraffin-embedded blocks and placed on silane-coated glass slides. The slides were washed in 100 mM of PB for 10 min and treated with proteinase K (5 μg/ml) for 10 min at 37°C, followed by refixation with 4% PFA in 100 mM of PB for 10 min at 27°C, and by acetylation with 100 mM triethanolamine, pH 8.0, and 0.25% glacial acetic acid. After washing twice with 100 mM of PB for 1 min each, the specimens were dehydrated through graded ethanol and air dried. Each specimen was hybridized with 100 ml of hybridization solution (50% formamide, 10 mM of Tris-HCl pH 7.6, 1 mM of EDTA, 600 mM of NaCl, 1 × Denhardt’s solution, 0.25% SDS, 10% Dextran sulfate and labeled probe) and incubated at 50°C for 16–20 hr in a moist chamber saturated with 50% formamide 4 × SSC. The specimens were then washed with 50% formamide 2 × SSC for 30 min at 50°C, twice with 2 × SSC for 15 min each at 50°C and twice with 0.2 × SSC for 15 min at 50°C. After washing briefly with 100 mM of Tris-HCl, pH 7.5, 150 mM of NaCl (TS) and 1.5% non-fat dry milk for 30 min at 27°C, the specimens were incubated with a 1:1000 dilution of alkaline-phosphatase conjugated anti-digoxygenin antibody (Boehringer Mannheim) for 45 min at 27°C, washed twice with TS for 15 min at 27°C, and briefly with 100 mM of Tris-HCl, pH 9.5, 100 mM of NaCl, 50 mM of MgCl2 (TSM). Development was carried out with Nitro-blue tetrazorium (175 ng/ml) and 5-Bromo-4-chloro-3-indolyl phosphate (340 ng/ml) in TSM for 3 hr at 27°C in the dark. After stopping the reaction with 10 mM of Tris-HCl, pH 8.0 and 1 mM of EDTA, the specimens were mounted with GEL/MOUNT (Biomedia, Foster City, CA) as described [12].
Cell migration assayThe underside of 24-well transwell inserts was coated with mouse vitronectin then blocked with heat-denatured albumin. The treated BMMs cultured in Teflon dishes were added to the top of the well and incubated overnight. Cells which had migrated to the underside of the well were counted by fixing in 2% formaldehyde, followed by staining with 1% methylene blue and counting under a microscope.
Treatment of murine BMMs with 1,25(OH)2D3 at a minimum effective dose of 10−8 M and with retinoic acid at a minimum effective dose of 10−7 M lead to clear changes in steady-state expression levels of β3 mRNA to as low as 10−9 M in a dose-dependent manner (Fig. 1A and B). By time-course assays, both 1,25(OH)2D3 and retinoic acid showed observable changes in steady-state levels of β3 mRNA as early as at 12 hr and maximum induction at 36 hr after the treatment (Fig. 1C and D).
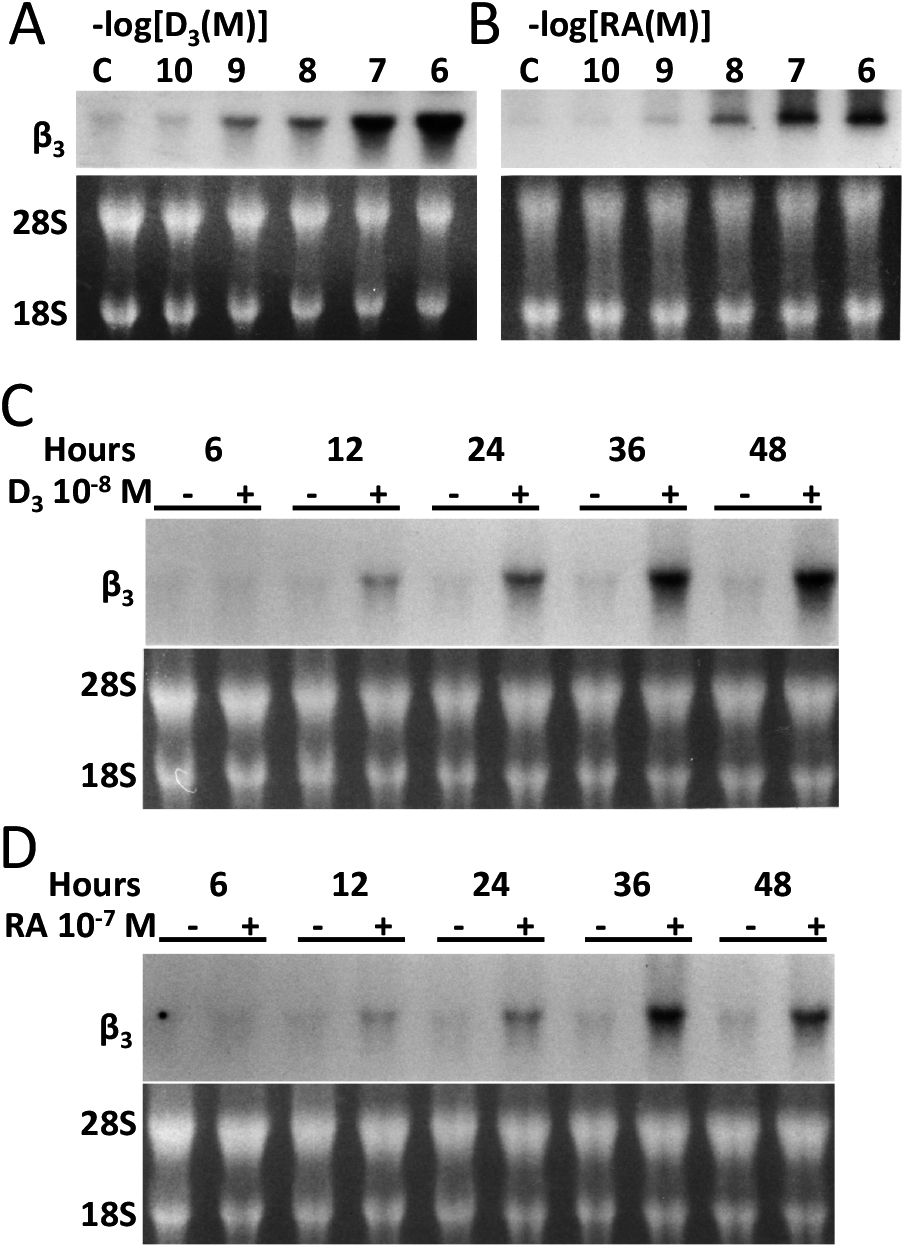
Minimal effective dose of 1,25(OH)2D3 or retinoic acid and time course of their treatment on murine BMMs and changes in the steady-state expression levels of β3 mRNA. After treatment of BMMs with various concentration of 1,25(OH)2D3 or retinoic acid, total RNA was isolated from BMMs and subjected to Northern blot analysis with the use of avian αv or murine β3 cDNAs. Equal loading is shown by the display of 28S and 18S RNA in the lower panels (C and D). Both 1,25(OH)2D3 and retinoic acid lead to changes in the steady-state levels of β3 in a dose-dependent manner at minimum effective dose of 1,25(OH)2D3 at 10−8 M and that of retinoic acid at 10−7 M (A and B). By time course assays, both 1,25(OH)2D3 and retinoic acid show maximum induction of β3 mRNA at 36 hr after the treatment (C and D).
Both 1,25(OH)2D3 and retinoic acid stimulated the surface expression of the integrin αvβ3 complex on murine osteoclast precursors (Fig. 2A). By Northern blot analysis, as demonstrated in Fig. 1, while both 1,25(OH)2D3 and retinoic acid augmented the steady-state expression of β3 integrin subunit mRNA, that of αv subunit mRNA remained constant (Fig. 2B).
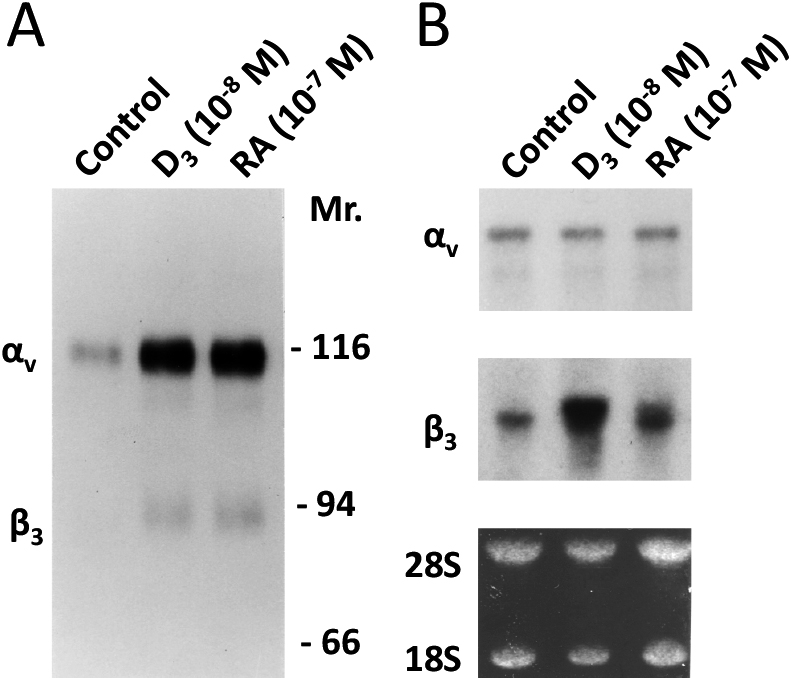
Both 1,25(OH)2D3 and retinoic acid stimulate expression of the integrin αvβ3 complex on murine osteoclast precursors by upregulating the steady-state expression of β3, but not of αv mRNA. BMMs cultured with the vehicle or with optimal concentrations of either 1,25(OH)2D3 or retinoic acid are surface-labelled and a lysate is immunoprecipitated with an anti-β3 polyclonal antibody. The immune complex was analyzed by SDS-PAGE/autoradiography. Both 1,25(OH)2D3 and retinoic acid upregulated the surface expression of integrin αvβ3 on murine osteoclast precursors (A). By Northern blot analysis shows both 1,25(OH)2D3 and retinoic acid augmented steady-state expression of β3, integrin subunit mRNA, while maintaining that of αv subunit mRNA unchanged (B).
IL-4 augments both 1,25(OH)2D3 and retinoic acid increases additively in steady-state expression of β3 mRNA levels at transcriptional level. These three agents increased the steady-state expression of β3 mRNA additively, while maintaining that of the αv subunit mRNA unchanged. The relative increase of β3 subunit mRNA expression against αv subunit mRNA expression by D3, RA, IL-4, D3 + RA, D3 + IL-4, RA-IL-4, D3 + RA + IL-4 was 0, 1.2, 0.6, 1.8, 6.2, 6.5, and 8.6, respectively (Fig. 3A). Mirroring Northern blot analyses, nuclear run-on assay showed that these three agents severally or jointly increased β3 mRNA at transcriptional level (Fig. 3B).
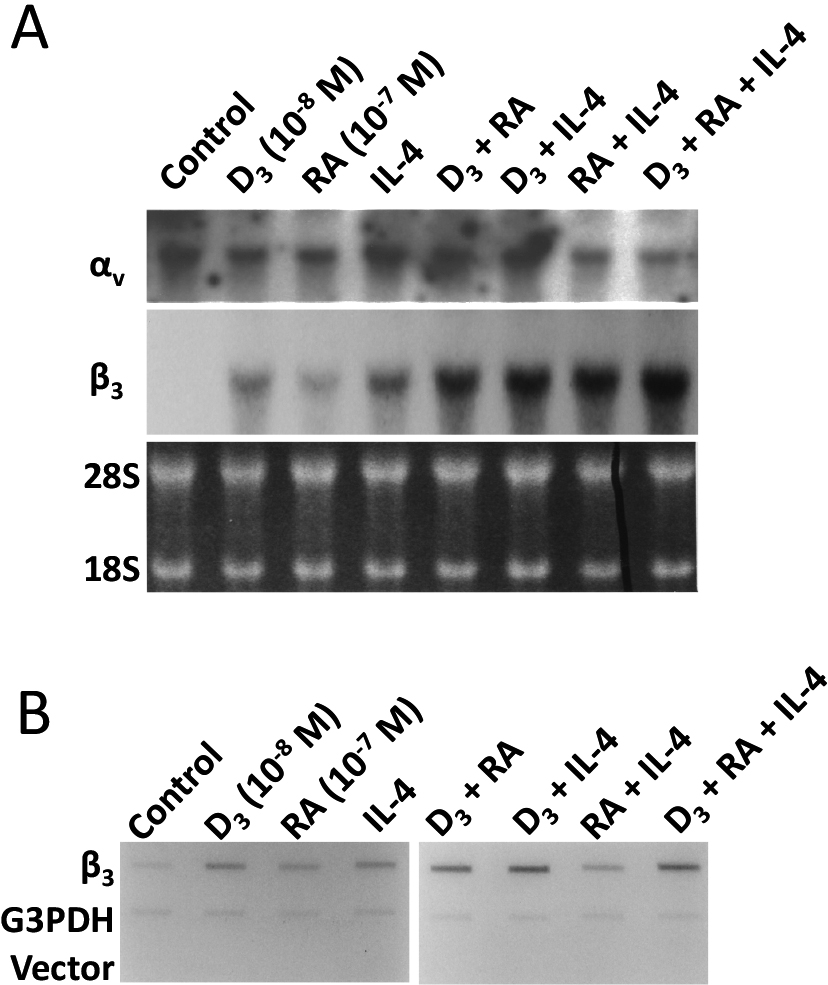
IL-4 augments both 1,25(OH)2D3 and retinoic acid additively in the steady-state expression of β3 mRNA levels at transcriptional level. Total RNA was isolated from cells treated with various combinations of IL-4, 1,25(OH)2D3 and retinoic acid, and subjected to Northern blot analysis. The three agents increased the steady-state expression of β3 mRNA, additively, while maintaining that of αv subunit mRNA unchanged (A). Nuclei from cells treated with various combinations of the cytokine and/or 1,25(OH)2D3 and retinoic acid were used to complete synthesis of nascent RNA in the presence of 32P-UTP. Total RNA was isolated and levels of labelled β3 mRNA were determined by nuclear run-on analyses. Mirroring the Northern blot analyses, nuclear run-on assay shows that these three agents severally or jointly increase β3 mRNA at transcriptional level (B).
By immunoprecipitation with an anti-β3 polyclonal antibody, the three compounds, severally or jointly, additively increased the surface expression of integrin αvβ3 on murine osteoclast precursors. The relative increase of β3 subunit by D3, RA, IL-4, D3 + RA, D3 + IL-4, RA-IL-4, D3 + RA + IL-4 was 6.4, 3.8, 6.2, 7.8, 6.8, 3.5, and 8.3, respectively, and was almost parallel to that of αv subunit (6.0, 3.2, 5.6, 7.0, 6.0, 3.2, and 6.4, respectively) (Fig. 4A). Mirroring surface-expressed integrin αvβ3, migration assay carried out with the use of transwells coated with vitronectin, revealed that BMMs treated with various combinations of the cytokine and/or the 1,25(OH)2D3 and retinoic acid displayed increased migration activity. The relative increase to the control (control = 1.0) in the number of migrating cells was then quantified (Fig. 4B).
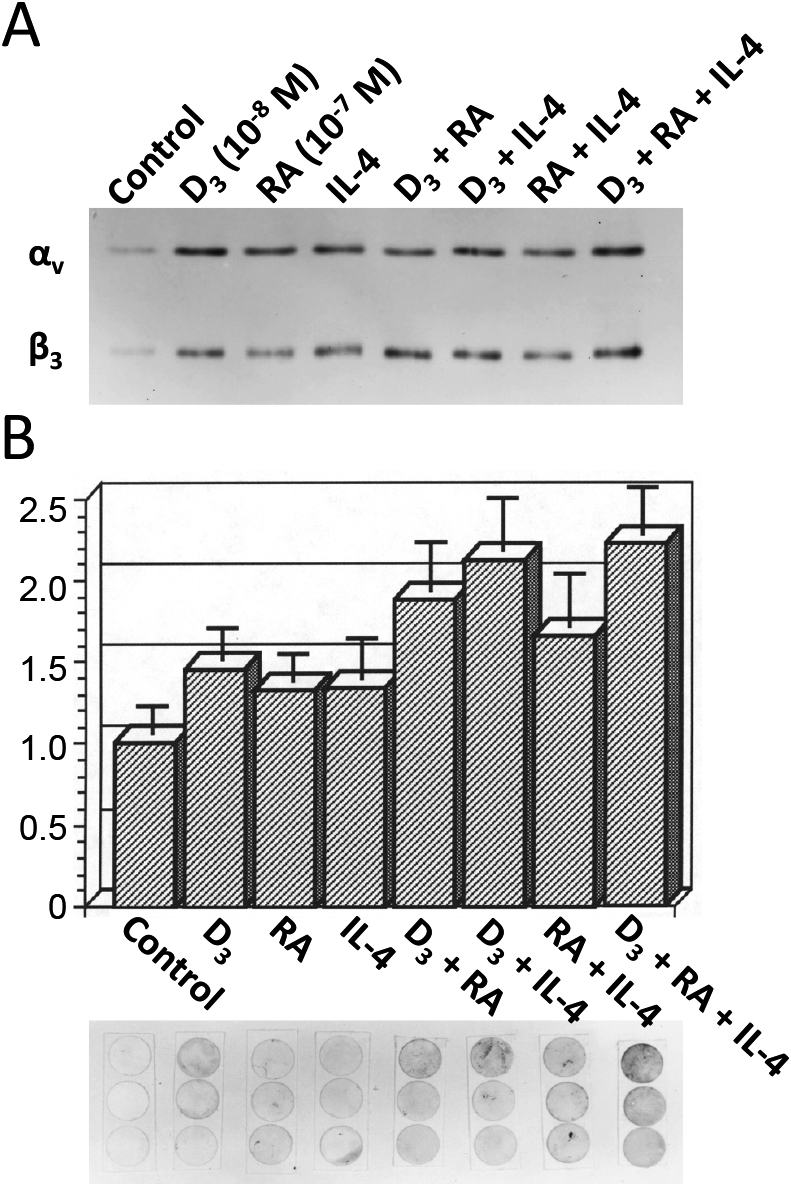
IL-4 augments both 1,25(OH)2D3 and retinoic acid additively the surface expression of the functional integrin αvβ3 on murine osteoclast precursors. BMMs treated with various combinations of the cytokine and/or 1,25(OH)2D3 and retinoic acid were surface-labelled with biotin, and a lysate immunoprecipitated with an anti-β3 polyclonal antibody. The immune complex was analyzed by SDS-PAGE/streptavidin peroxidase complex. The agents severally or jointly increase surface expression of integrin αvβ3 on murine osteoclast precursors, additively (A). BMMs treated with various combinations of the cytokine and/or 1,25(OH)2D3 and retinoic acid were placed in a migration assay as described in Materials and Methods, and the relative increase in the number of migrating cells was quantified. The lower panel shows membranes of three independent migration assays stained with methylene blue (B). Vertical value is a relative increase to the control (control = 1.0) in the number of migrating cells in each treatment.
In situ hybridization revealed that the expression of the β3 integrin subunit mRNA in BMMs treated with 1,25(OH)2D3 at 10−8 M (Fig. 5C) and with retinoic acid at 10−7 M (Fig. 5E) was significantly higher than that in vehicle-treated BMMs (Fig. 5A). Not much difference from the viewpoint of the cell shape of BMMs was observed among treated and non-treated cells, and the expression pattern of the β3 integrin subunit mRNA was even in the cytoplasm. On the other hand, BMMs treated with IL-4 showed the formation of numerous multinucleated giant cells (arrows in Fig. 5G), where the expression of the β3 integrin subunit mRNA was prominent. Negative controls prepared by sense-probe showed no significant signals (Fig. 5B, D, F and H).
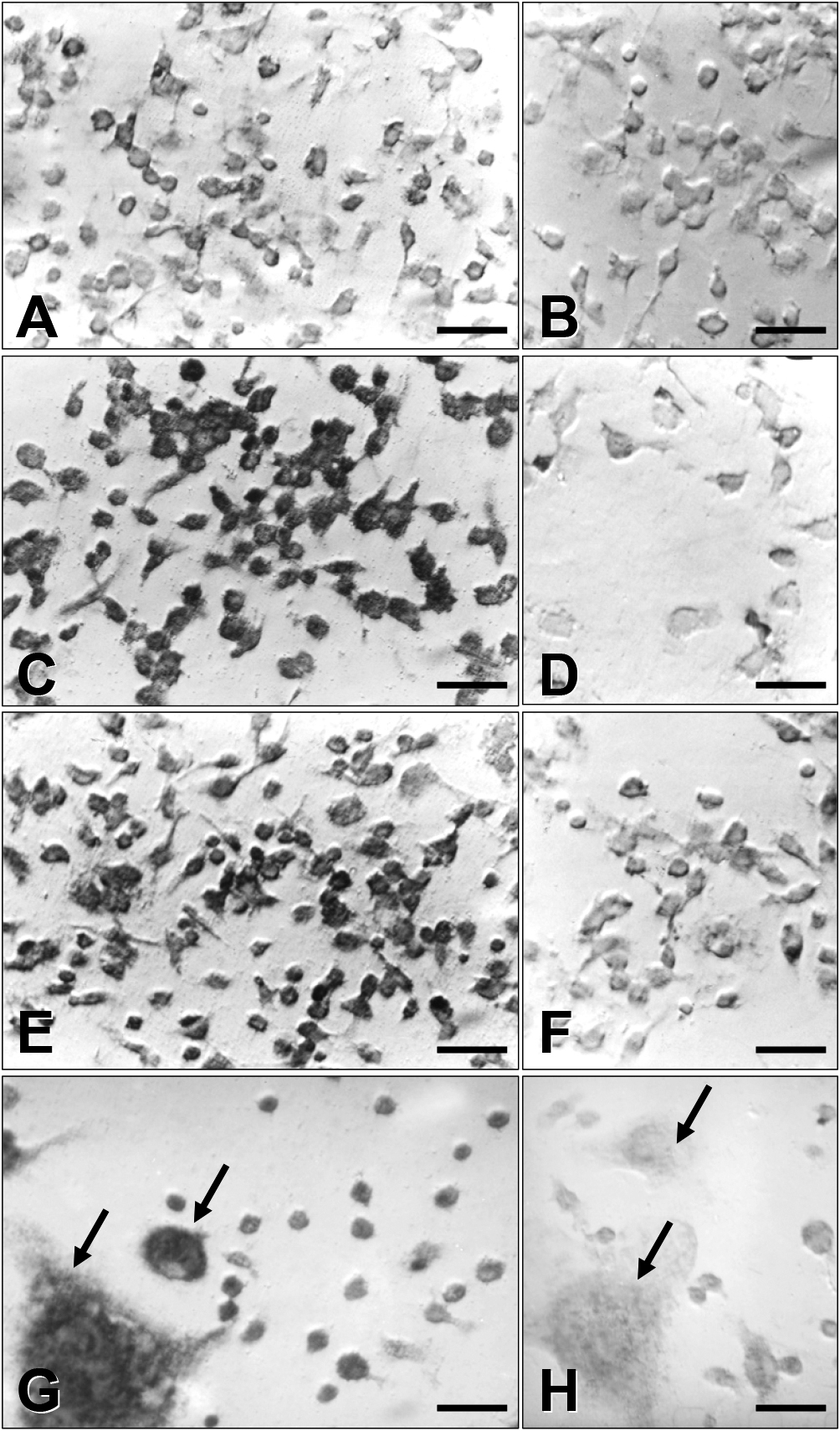
Morphological analyses of β3 mRNA expression in BMMs by in situ hybridization. BMMs treated with optimal concentration of either 1,25(OH)2D3 or retinoic acid were fixed and processed for in situ hybridization with the use of single-stranded antisense and sense β3 cDNA probes. A, C, E and G: antisense β3 cDNA probe, and B, D, F and H: sense β3 cDNA probe. A and B: vehicle-treated cells. C and D: 1,25(OH)2D3-treated cells. E and F: retinoic acid-treated cells. G and H: IL-4-treated cells with multinucleated giant cells. Both 1,25(OH)2D3 and retinoic acid and IL-4 treatment increase cytoplasmic expression of β3 mRNA. Bar = 50 μm.
To study in vivo distribution of β3 mRNA expression in bone, histo-in situ hybridization was additionally carried out for tibia from normal and IL-4 transgenic mice. In normal mouse tibia (Fig. 6A), β3 mRNA expression was detected on mature osteoclasts around the trabecular bone surface, and on megakaryocytes in the bone marrow milieu. The negative control was examined by a sense β3 cDNA probe. In tibial bone from the IL-4 transgenic mouse (Fig. 6B), trabecular bone volume was markedly reduced to osteoporotic as described [17], and the number of β3 mRNA-positive osteoclasts lining the trabecular bone surface was also reduced, while the number of megakaryocytes was maintained. Negative controls prepared by the sense β3 cDNA probe did not show significant signals (Fig. 6A and B).
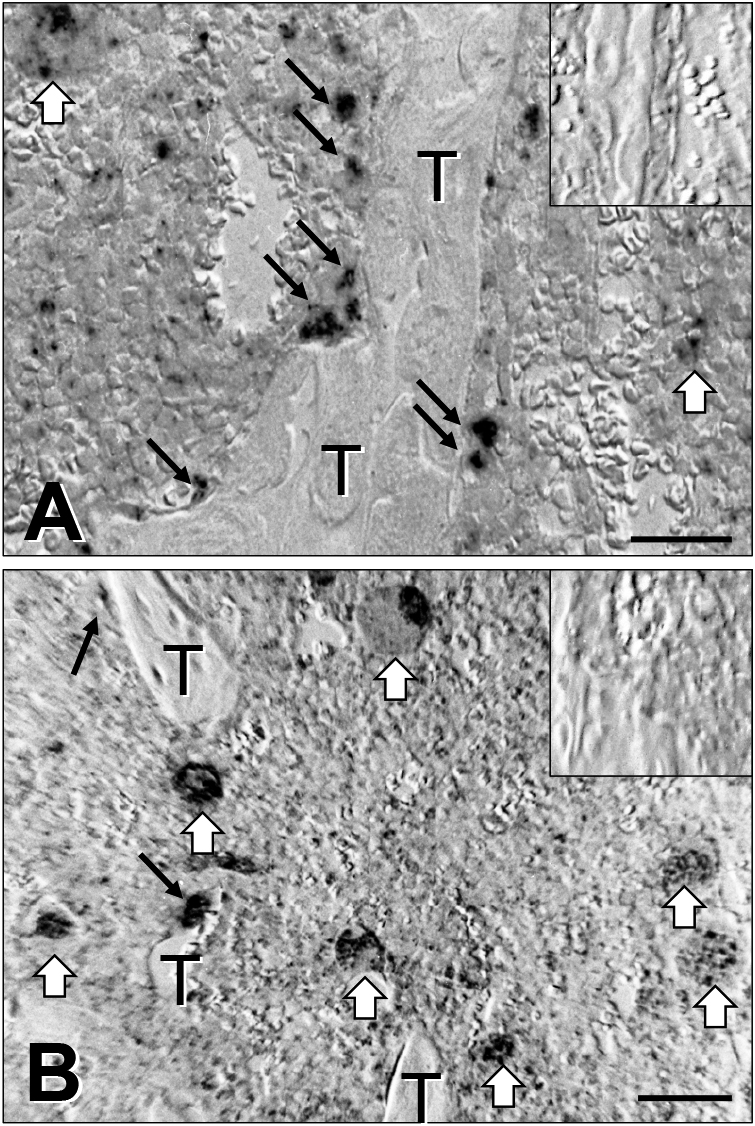
Distribution of β3 mRNA expression on tibia from normal and IL-4 transgenic mice by in situ hybridization. Tibiae were removed from normal and IL-4 transgenic mice, fixed and decalcified for in situ hybridization with the use of single-stranded antisense and sense β3 cDNA probes. In normal mouse tibia (A, ISH), β3 mRNA expression is observed on osteoclasts (black arrows) around trabecular bone (T), and megakaryocytes (open arrows). The inset in the upper right corner is a negative control probed by sense β3 cDNA. In tibial bone from the IL-4 transgenic mouse (B, ISH), trabecular bone (T) volume is reduced, and the number of β3 mRNA-positive osteoclasts is reduced (black arrows), while that of megakaryocytes (open arrows) is maintained. The inset in the upper right corner is a negative control probed by sense β3 cDNA. Bar = 100 μm.
Osteoclasts or their precursors express three major integrin subunits, namely β1, β3, and β5 [26]. Among these, β3 is regarded as the major attachment molecule that regulates osteoclast function [17, 26, 31]. Indeed, the β3 integrin subunit knockout mouse is osteosclerotic because of dysfunctional osteoclasts [8, 19]. Although expressed in the early stages of osteoclastic differentiation, the β5 subunit, 56% homologous to β3, that binds solely to the αv subunit, also contributes to osteoclast differentiation and function [5]. In our current study, cytokine IL-4, 1,25(OH)2D3 and retinoic acid increased the expression of integrin β3 in murine BMMs as demonstrated by Northern blot and in situ hybridization studies. The nuclear run-on study showed that all three stimulated transcription of the β3 gene by transactivating it in an additive manner. Overall, our results indicate that cytokines, 1,25(OH)2D3 and retinoic acid modulate αvβ3 expression in murine osteoclast precursors through mechanisms involving transactivation of the β3 gene. In the tibiae of the IL-4 transgenic mouse, however, while megakaryocytes showed strong signals of the β3 integrin subunit, the number of BMMs and mature osteoclasts expressing these signals was markedly reduced (Fig. 6B). This apparent inconsistency between in vitro studies of BMMs and the IL-4 transgenic mouse may be explained by the fact that osteoporosis or osteopenia in the IL-4 transgenic mouse is mainly due to the impairment of osteoblastic maturation [18, 27], and that receptor activator of NF-κB ligand (RANKL) expressed on osteoblastic cells, requisite for osteoclastogenesis [29], is, thereby, impaired. Moreover, the reduced number of osteoclasts in the IL-4 transgenic mouse may be explained by the fact that IL-4 prompts these BMMs along an antigen-presenting phagocytic pathway and away from the osteoclast phenotype [4]. Therefore, artificial and forced-induction of the β3 integrin subunit on BMMs, cells at an early stage of osteoclast precursors, by bone-seeking steroids or cytokines, does not always favour osteoclastogenesis, and sequential expression of integrins during osteoclastogenesis is essential for proper osteoclastic differentiation.
A model has been developed in which the expression of a given integrin is mediated by one of its subunits [30]. In the case of αvβ3, we and others have shown that β3 plays this role [17, 22]. Indeed, this study confirmed that it is the β3 subunit that defines the surface expression of integrin αvβ3. Moreover, these changes are functional, as indexed by increased capacity of treated cells to migrate in a chemotactic assay. IL-4 is an immunoregulatory cytokine produced by T-lymphocytes and mast cells, exerting an array of effects on mononuclear phagocytes, and provoking macrophage precursor differentiation along an immunoregulatory pathway into cells primed to participate in an inflammatory response [1, 23, 24]. Indeed, IL-4 overexpression in transgenic mice exhibits a peculiar mononuclear inflammatory ocular lesion [27]. In this regard, by sequestering monocyte-macrophage populations from osteoclast precursors, IL-4 is apparently anti-osteoclastogenic in vitro as described [17]. This is true to the in vivo model; bone remodelling, a process initiated by osteoclastic activity, is markedly reduced in IL-4 transgenic mice [18, 27]. Taken together, the data that αvβ3 mediates phagocytosis of cells undergoing apoptosis [28], that IL-4 induces macrophage polykaryons from common precursors of osteoclasts [13], and thus reducing the number of osteoclasts, IL-4 may be important to the process of scavenging cell debris and, at the same time, tightening bone around inflammatory lesions within a limited period of time. Long-term continuous overexpression of IL-4 in transgenic mice, however, may result in a low-turnover osteopenia state by reducing both osteoblastic and osteoclastic activities.
In conclusion, our data indicate that while surface expression of functional αvβ3 expression, which is regulated by the β3 subunit, is a necessary component of osteoclastic bone resorption, sequential and regulated induction of the specific integrin at appropriate stages is essential for accomplishing proper macrophage differentiation into osteoclasts.
Kitazawa S performed the majority of experiments, analyzed the data and wrote the paper; Haraguchi R, Kohara Y and Kitazawa R analyzed the cell cultures.
The authors declare that there are no conflicts of interest.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (16H05161 to S.K., 18K06832 to R.H., 17K18438 to Y.K., and 15K08426 to R.K).