2020 Volume 53 Issue 6 Pages 147-155
2020 Volume 53 Issue 6 Pages 147-155
Minamata disease is a methylmercury poisoning caused by consumption of marine food contaminated by man-made methylmercury environmental pollution, and its most prominent feature is marked pathological changes in the central nervous system. Morphological alterations are less pronounced in the liver and the kidney, although their mercury levels are higher than those of the brain. In marine mammals, methylmercury is known to be easily converted to inorganic mercury and it combines with selenium forming mercury selenide, which may counteract the toxicity of mercury. However, little is known about the formation of mercury and selenium complex in human organs. In the present study, we examined the cerebrum, cerebellum, liver, and kidney of a Minamata disease case to study the mercury and selenium localization using electron probe microanalysis. Our results indicated the mercury and selenium localization in the specified tissue of the brain, liver, and kidney such as glial cells, Kupffer cells, and renal tubules.
Minamata disease was caused by ingestion of fish and shellfish contaminated with high levels of methylmercury (MeHg) [16]. The most prominent feature of Minamata disease is significant pathological changes in the central nervous system [16]. Although mercury levels the liver and kidney are higher than those in the central nervous system, the pathological changes are not as pronounced [16]. The patients show the lesions in the cerebellar cortex, calcarine cortex, precentral gyrus, postcentral gyrus, and transverse temporal gyrus [16]. Histopathologically, a characteristic loss of neurons with gliosis in the cortical II and III layers is seen in the cerebrum regardless the severity. The characteristic cerebellar changes are loss of granular cells, and the relatively well-preserved Purkinje cells [16].
Although the mercury in the organs are predominantly MeHg in the early stages of the MeHg exposure, the proportion of inorganic mercury (I-Hg) increases gradually with time [8, 18]. Therefore, in both the acute and subacute cases, the proportion of MeHg to total mercury (T-Hg) in the body is high, but the proportion becomes low in the chronic cases. Takeuchi and Eto [14] reported that the average T-Hg and MeHg concentrations in the brain of the acute cases were 12.6 and 5.61 ppm, and those of the long-term follow-up cases were 3.16 and 0.24 ppm, respectively.
Ganther et al. [5] demonstrated that the selenium (Se) played the protective role against the MeHg toxicity in the studies using marine mammals and experimental animals. The concentrations of T-Hg, I-Hg, MeHg, and Se in the organs of Niigata Minamata disease patients have been reported [3], and a strong correlation between mercury and Se concentrations has been shown in the liver [3].
In clinical human samples, the existing of I-Hg was reported using photoemulsion and autometallography [2, 10]. In Minamata disease cases, the localization of I-Hg was visualized by photoemulsion [15]. However, the histological localization of MeHg in the central nervous system and various organs has not been elucidated, because the method of the MeHg visualization is not developed. In addition, the staining method of Se, which may decrease the MeHg toxicity by forming a chemical complex with I-Hg, has not been reported.
Electron probe microanalysis (EPMA) captures the distribution of metallic elements in tissue sections [6, 17], and has been used to detect the distribution of relatively high concentrations of elements in diseases such as primary biliary cirrhosis [6], Wilson’s disease, and occupation-related lung diseases [13, 17], as well as in the skeletal muscle of dolphins [11]. In this study, we used EPMA to examine the localization of mercury and Se in tissues from a Minamata disease case.
This patient was a Japanese male in his 30s who died in 1958. He had experienced numbness in the upper limbs for approximately 30 mo prior to his death, but did not receive treatment. Approximately 20 days before death, he developed symptoms characteristic of Minamata disease and was diagnosed clinically. As controls, 3 cases other than Minamata disease, which were dissected in 1958–1960, were used. This study is described in accordance with guidelines on patient information protection in the case report of the Japan Pathological Association, and was approved by the National Minamata Disease Research Center Research Ethics Review Committee.
Preparation and staining of tissue specimensBlocks of paraffin-embedded were prepared shortly after the patient’s death. Blocks of cerebral calcarine cortex, cerebellum, liver, and kidney sections were sliced into 3-μm serial sections with a microtome and stained with hematoxylin and eosin. The sections were subjected to autometallography [2].
EPMA analysisSerial sections were affixed to a carbon sample stage (Niigata Science, Niigata, Japan), deparaffinized, and dried. The specimens were sputter-coated with carbon prior to elemental analysis. Scanning electron microscopy was performed to assess morphological changes, and energy-dispersive X-ray spectroscopy was conducted to determine the elemental composition using an electron probe microanalyzer (JXA-8530F, JEOL, Tokyo, Japan) with an acceleration voltage of 25 kV. Next, we used EPMA for elemental mapping of mercury and Se with 256 × 256 pixel mapping. The accelerating voltage and beam current were set to 15 kV and 0.6 μA, respectively.
Measurement of mercury concentrationsFormalin-fixed liver, kidney, cerebrum and cerebellum were used for analysis. T-Hg concentrations were determined by cold vapor atomic absorption spectrophotometry according to a previously described method [1]. MeHg concentrations were measured by the Akagi method [1]. I-Hg was calculated as T-Hg minus MeHg. Se concentrations were measured using an inductively coupled plasma mass spectrometer equipped with a collision cell. T-Hg in standard reference material, DORM-2 (Dogfish Muscle; National Research Council, Ottawa, Canada), was measured as quality control, and the measured results fell within the certified range of 4.64 ± 0.26 μg/g. Dogfish muscle DORM-2 was used as the standard reference material for MeHg determinations, and the measured results fell within the certified range of 4.47 ± 0.32 μg/g. NIST 1577 (Bovine Liver; Gaithersburg, USA) was used as a quality control of Se measurement, and the obtained results fell within the certified range of 0.73 ± 0.06 μg/g.
We observed necrosis of cortical layer II to IV neurons and neuropil degeneration in the cerebrum (Fig. 1A, 1B). In the cerebellum, loss of granule cells beneath the Purkinje cell layer and proliferation of the glial cells were observed (Fig. 1C). There were no significant changes in the liver and kidney (Fig. 1F, 1G). Autometallography detected I-Hg in the microglial cells of the calcarine cortex (Fig. 2A) and the glial cells of the cerebellum (Fig. 2B). Multiple I-Hg granules were observed in the hepatocytes and Kupffer cells (Fig. 2C). In the kidney, I-Hg was detected in the epithelial cytoplasm of the proximal and distal renal tubules (Fig. 2D).

Photomicrographs of calcarine cortex and cerebellum from a Minamata disease patient and control. In the cerebrum, necrosis of cortical layer II to IV neurons and neuropil degeneration (A, B). Loss of granule cells beneath the Purkinje cell layer and proliferation of the glial cells were observed in the cerebellum (D). In the control case, no significant changes were observed in the cerebrum (C) and cerebellum (E). There were no significant morphological changes in the liver (E) or kidney (F) of a Minamata disease patient.

Photomicrographs of autometallography analysis of calcarine cortex, cerebellum, liver, and kidney tissues from a Minamata disease patient. In the cerebrum, multiple inorganic mercury granules were observed in the microglial cells of the calcarine cortex (A). In the cerebellum, inorganic mercury was detected in the glial cells (B). In the liver, mercury was detected in hepatocytes and Kupffer cells (C). In the kidney, mercury was detected in the epithelial cytoplasm of the proximal and distal tubules (D).
Scanning electron microscopy showed white granular materials that indicated metal aggregation (Fig. 3A) in all organs tested; these white granules contained mercury and Se (Fig. 3B). No evidence of metal aggregation was observed in the organs of control cases.
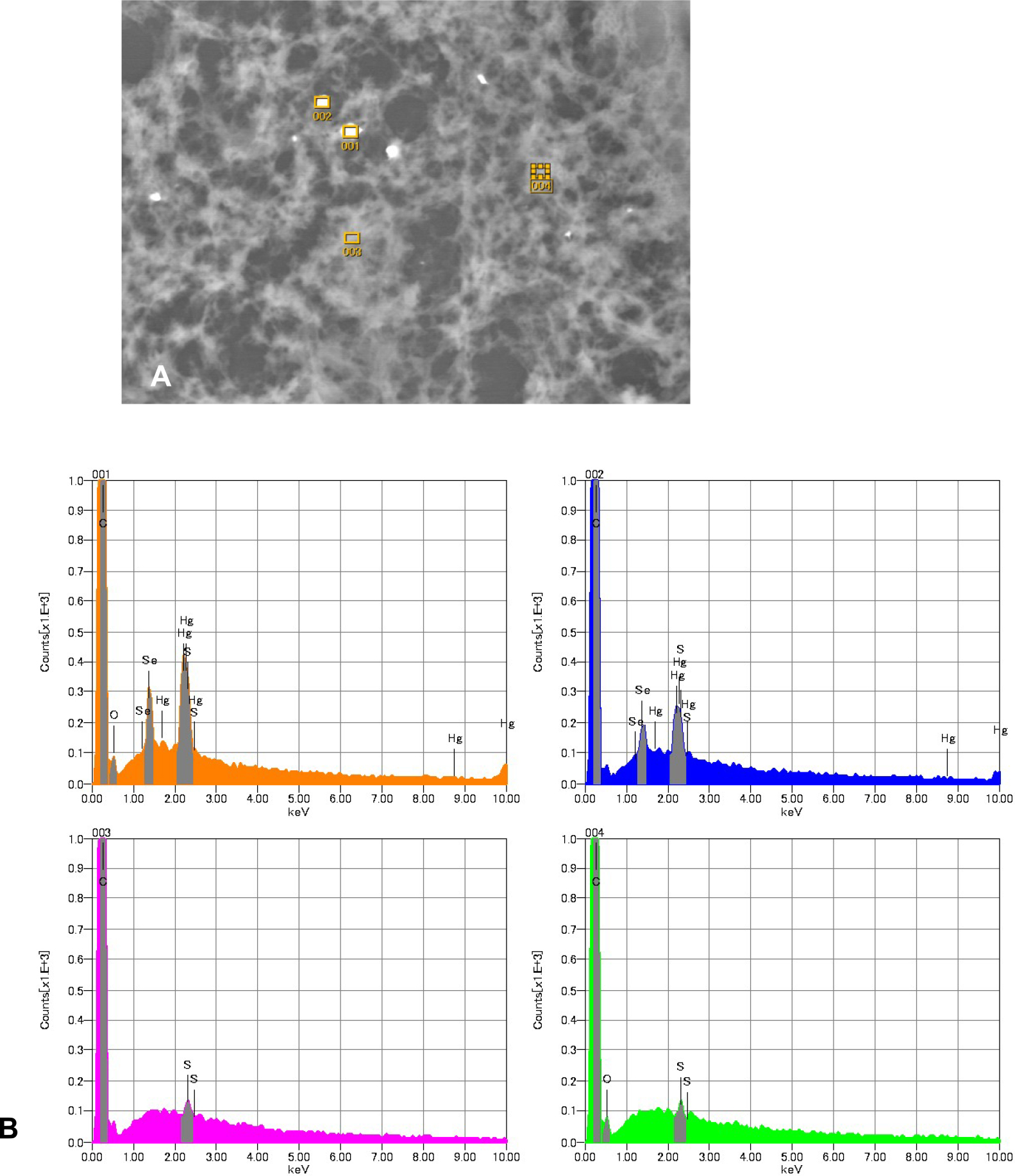
(A) Scanning electron microscopy showed white granular materials, indicating metal aggregation in the kidney. (B) Energy-dispersive X-ray spectroscopy showed that these white granules contained mercury and selenium (001, 002). Mercury and selenium were not detected in areas lacking white granules (003, 004).
Mercury was detected in the glial cells of the calcarine sulcus; some glial cells also contained Se (Fig. 4B, 4C). Neither mercury nor Se were found in the medulla. Mercury and Se were detected in the glial cells in the cerebellum (Fig. 4E, 4F). Mercury and Se were detected in Kupffer cells and hepatocytes; concentrations were higher in Kupffer cells (Fig. 4H, 4I). In the kidney, high concentrations of mercury and Se were found in the proximal and distal tubules, and their distribution was almost identical. None were found in glomeruli or the collecting ducts (Fig. 4K, 4L). Detection of mercury and selenium was confirmed in the organs of Minamata disease cases other than this case (data not shown).

Compositional image in backscattered electron mode (A, D, G, J) and electron probe microanalysis mapping of mercury (B, E, H, K) and selenium (C, F, I, L). (A–C) Calcarine cortex. Mercury was detected in the glial cells of the calcarine sulcus; selenium was detected in some glial cells. Neither mercury nor selenium were detected in the medulla. (D–F): Cerebellum. Mercury and selenium were detected in the glial cells. (G–I): Liver. Mercury and selenium were detected in Kupffer cells and hepatocytes, particularly in Kupffer cells. (J–L): Kidney. Mercury and selenium were present in the proximal and distal tubules, and their distribution was almost identical. None were detected in glomeruli or collecting ducts.

Continued.
Table 1 lists mercury and Se concentrations in the organs tested. Relatively high concentrations of mercury were detected in the liver and kidney, mostly in the form of I-Hg. Although the mercury concentration in the brain was lower than in the liver and kidney, approximately 50% of it was MeHg.
| Tissue | Total mercury | Methylmercury | Inorganic mercury | Selenium |
|---|---|---|---|---|
| Cerebrum | 13.8 | 5.00 | 8.80 | 0.85 |
| Cerebellum | 12.8 | 6.00 | 6.80 | 0.98 |
| Liver | 46.2 | 8.45 | 37.8 | 20.8 |
| Kidney | 111 | 10.0 | 101 | 12.0 |
Units are microgram per gram of wet weight.
To our knowledge, this is the first report which indicated the mercury and selenium localization in the specified tissue of the brain, liver, and kidney such as glial cells, Kupffer cells, and renal tubules using an EPMA method.
In this case, lesions characteristic of Minamata disease were observed in the cerebrum and cerebellum, but inorganic mercury and selenium were distributed in diffuse glial cells regardless of the severity of the lesion. In the liver and kidney, mercury and Se were distributed in the same locations, suggesting that I-Hg converted from MeHg makes a mercury and selenium complex which is presumably the mercury selenide (HgSe). However, the formation of HgSe has not been reported in patients of MeHg poisoning.
Selenium has been shown to protect against MeHg toxicity in animal experiments [7, 12]. In dolphin, ingested MeHg binds to various compounds (proteins), and converts to I-Hg and to an insoluble HgSe mineral termed tiemannite [9]. We hypothesized that the formation of HgSe may play a role for Se protection against MeHg poisoning in humans, too.
HgSe has been detected in the brain of several cetacean species, suggesting that MeHg may be demethylated and converted to HgSe in the brain [4, 9]. The HgSe formation may also happen in humans. Our result showed that Se was co-localized in some cerebral microglial cells where mercury deposition was observed. However, only mercury was detected in some microglial cells, suggesting the presence of chemical forms of mercury other than HgSe.
Inorganic Hg has been shown to exert toxic effects on the kidney [19]. Although the kidney concentration of I-Hg in our sample was high (101 ppm), no lesions were observed histopathologicaly, suggesting a protective effect by Se. In this study, high concentrations of mercury and Se were detected in the renal proximal tubules and distal tubules. In cetaceans, the liver is known to demethylate MeHg [7]. Rather than being demethylated in the renal tubular epithelial cells, it is likely that MeHg demethylated to I-Hg in the liver was reabsorbed by the tubular epithelial cells and bound to Se.
The EPMA analysis method developed by Watanabe does not discuss the detection limit [17]. Since the method uses paraffin-embedded sections, it is thought that some degree of metal aggregation is required. Therefore, it is considered that whether or not the presence or absence of local aggregation can be detected is determined rather than the concentration in the entire organ.
We analyzed the samples which were derived from a Minamata disease patient who was autopsied in 1958, and our results indicated the localization of mercury and selenium in the brain, liver and kidney. This study also suggest that even paraffin blocks stored for decades can still be useful to provide new histopathological aspects using a modern techniques of analysis.
The authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
MM and MS contributed to the study design; MM, KM, and ST acquired, analyzed, and interpreted the data; MM drafted the manuscript; MS, KM, ST, and YK critiqued and revised the manuscript. All authors approved the final draft and agreed to be accountable for all aspects of the work, to ensure that questions relating to the accuracy or integrity of any part of the work would be appropriately investigated and resolved.
The authors are grateful to Dr. Yoshifumi Takahashi, Nanae Ryuzaki and Satsuki Nagase, Department of Dental Material Science, Dentistry School of Aichi Gakuin University, Nagoya, Japan. We want to thank Mao Uchikakoi, Miwa Chijiiwa and Ai Motoyama, National Institute for Minamata Disease.