2022 Volume 55 Issue 1 Pages 47-56
2022 Volume 55 Issue 1 Pages 47-56
Gamma-aminobutyric acid (GABA) is an inhibitory neurotransmitter in the mature brain; however, it acts excitatory during development. This difference in action depends on the intracellular chloride ion concentration, primarily regulated by potassium chloride co-transporter2 (KCC2). Sufficient KCC2 expression results in its inhibitory action. GABA is also abundant in pancreatic islets, where it acts differentially on the islet cells, and is involved in carbohydrate metabolism. However, the mechanisms underlying the differential action remain unknown. We performed immunohistochemistry for glutamic acid decarboxylase (GAD), a synthetic enzyme for GABA, and KCC2 in normal adult islets. GAD was co-localized with insulin in β cells, whereas KCC2 was expressed in glucagon-positive α cells. These results are in line with previous observations that GABA decreases glucagon release but increases insulin release, and suggest that GABA and insulin may work together in reducing blood glucose levels under hyperglycemia. Next, we examined the streptozotocin-induced type1 diabetes mellitus mouse model. GAD and insulin expression levels were markedly decreased. KCC2 was expressed in glucagon-positive cells, whereas insulin- and somatostatin-positive cells were KCC2-negative. These findings suggest that in diabetes model, reduced GABA release may cause disinhibition of glucagon release, resulting in increased blood sugar levels and the maintenance of hyperglycemic state.
In the mature central nervous system (CNS), γ-aminobutyric acid (GABA) is a major inhibitory neurotransmitter that mediates hyperpolarization of membrane potential and negatively regulates neuronal activity [25, 29]. In contrast, in the immature CNS, it causes membrane potential depolarization [2, 3, 28, 30, 36]. The difference in action depends on the intracellular chloride ion concentration ([Cl−]i) [16, 22, 26, 37, 44].
GABA is also localized in non-neural organs, such as the thyroid, lung, heart, liver, spleen, small intestine, kidney, pancreas, and uterus [12]. GABA is notably abundant in pancreatic islets, which are involved in carbohydrate metabolism [35, 42]. The pancreatic islets consist of three types of endocrine cells—α, β, and δ cells—that specifically release the hormones glucagon, insulin, and somatostatin, respectively. Glucagon elevates blood glucose level, whereas insulin decreases it. GABA is synthesized by glutamic acid decarboxylase (GAD) in β cells [6, 7, 35]. Antibodies against GAD are frequently detected in the plasma serum of type 1 diabetes mellitus patients (T1DM), and their islet β cells are destroyed by an autoimmune response [1, 27]. GABA is released from β cells together with insulin through exocytosis [14, 15, 32], and plays roles in controlling the release of glucose-regulating hormones [9, 14, 19, 38, 40, 48]. GABA increases insulin release from β cells [8, 32, 41], whereas it decreases glucagon release from α cells via membrane hyperpolarization [39, 49, 50]. Previous studies suggest that the differential action of GABA on α and β cells may depend on the [Cl−]i [5, 10], as in the CNS [2, 30, 31]. Davies et al. first demonstrated the localization of potassium chloride co-transporters (KCCs), which transport Cl− out of cells and decrease [Cl−]i in islet cells [10]. However, they did not clearly determine their cellular localization and the type of KCCs and in the islets, because the antibody specificity was not clearly evaluated. Recently, Kursan et al. demonstrated that KCC2, that is one of the KCCs and abundant in the CNS [2, 30, 31], is localized in most pancreatic islet cells including α, β, and δ cells, using a polyclonal antibody [21]. When KCC2 is abundantly expressed, [Cl−]i is low, and GABA may also function as an inhibitor to β cells as well as α cells. Thus, their findings were not in agreement with other previous reports [9, 14, 19, 38, 40, 48]. Furthermore, in the supplementary figures, the paper also showed the inconsistent immunohistochemical staining that KCC2 expression was restricted in the marginal part of the islets using another antibody [21]. Both antibodies were raised against the same epitope region of KCC2 protein, suggesting that the KCC2 localization may not yet be determined. Thus, the mechanisms underlying the differential action of GABA on individual islet cells remained unclear.
In this study, to clarify the role of GABA on islet cells, we performed immunohistochemistry for GAD and KCC2 in the pancreatic islets. In the CNS, as expression of KCC2 frequently changes during development and after nerve injury, KCC2 may play a key role in the fine tuning of [Cl−]i, and may thus be a key regulator of the action of GABA [16, 22, 26, 37]. A similar regulatory system may be present in islet cells. In addition, to elucidate the various GAD- and KCC2-expressing cell types, we co-stained for glucagon, insulin, or somatostatin. Finally, to provide insight into the changes in GABAergic signaling in islets in T1DM, we examined the changes in the localization of KCC2 and GAD in the mouse model of streptozotocin (STZ)-induced diabetes [18, 20, 24].
Male C57BL/6J mice, 10–12 weeks of age (SLC, Shizuoka, Japan), were maintained at a controlled temperature (24 ± 2°C) and humidity (50 ± 10%) under a 12/12-hr light/dark cycle, and fed a standard diet. The procedures in this study were approved by the Animal Care and Use Committee of the University of the Ryukyus (Approval No. A2019197), and all animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of University of the Ryukyus. Protocols for the care and handling of animals conformed with current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996). Every effort was made to minimize the number of animals used and their suffering. For immunohistochemistry for GAD and KCC2, four mice were used.
Production of the mouse model of T1DMSTZ was intraperitoneally injected (200 mg/kg body weight) at day 0 (D0) and D3 as previously reported [18, 20, 24]. Body weight and blood glucose concentration were measured on D7. Whole blood was taken from the tail vein, and blood glucose concentration was measured using an automatic glucometer (Medisafe Mini; Terumo, Tokyo, Japan) [20]. Nine mice were injected with STZ to generate T1DM model mice, and 10 mice were used as controls. Body weight and blood glucose concentration in control and STZ-induced mice were analyzed using Student’s t-test. A value of p < 0.05 was statistically significant.
AntibodiesAntibodies used in this study are listed in Table 1. Antibodies against GAD and KCC2 are our original antibodies, and their specificities had already checked by Western Blotting and absorption test [43, 45].
| Antigen | Immunogen | Manufacturer, species, antibody type | Dilution/Final concentration |
|---|---|---|---|
| GAD | Synthetic peptide of the C terminal of mouse GAD with the amino acid sequence [C]DFLIEEIERLGQDL | Original antibody, guinea pig, polyclonal [43] | 1 μg/mL |
| GAD | Synthetic peptide of the C terminal of mouse GAD with the amino acid sequence [C]DFLIEEIERLGQDL | Merck, Cat. No. AB1511, Lot No. 2580693, Rabbit, polyclonal | ×250 |
| Glucagon | Polymerised porcine glucagon | SIGMA, Cat. No. G2654, Lot No. 112M4804, Mouse monoclonal | ×100 |
| Insulin | Synthetic peptide corresponding to the sequence of human insulin | Cell Signaling TECHNOLOGY, Cat. No. 4590, Lot No. 2 Rabbit polyclonal | 1 μg/mL |
| KCC2 | Synthetic peptide, aa 44–64 from N-terminals of mouse | Original antibody, guinea pig polyclonal [45] | 1 μg/mL |
| Somatostatin | Somatostatin conjugated to BSA | MILLIPORE, Cat. No. AB5494, Lot No. 2342864 Rabbit polyclonal | 2 μg/mL |
Under deep anesthesia induced by intraperitoneal injection of a mixed solution (10 μL/g body weight) of 8% (v/v) Somnopentyl (pentobarbital sodium, 5 mg/mL, Kyoritsu Seiyaku, Tokyo, Japan) and 20% (v/v) ethanol in saline, normal control mice and STZ-injected mice on D7 were transcardially perfused with 4% (v/v) paraformaldehyde in phosphate buffer (PB; 0.1 M, pH 7.4). The pancreas with the duodenum was immersed in the same fixative overnight, cryoprotected with 30% (w/v) sucrose in PB for 48 hr at 4°C, and cut into sections (20 μm thickness) using a cryostat (Leica Biosystems, Nussloch, Germany), and then mounted on gelatin-coated glass slides.
ImmunohistochemistryFor the single staining, sections on glass slides were treated with 100% (v/v) methanol containing 0.3% (v/v) H2O2 for 30 min, followed by PB for 10 min, 3% (v/v) normal goat serum in PB for 1 hr, and then guinea pig antibodies against GAD or KCC2 at room temperature. After rinsing three times with PB for 15 min, sections were visualized using the avidin-biotin-peroxidase complex (ABC) method with a Histofine kit (Nichirei, Tokyo, Japan). To test the specificity of each antibody, each peptide used for the immunization [43, 45] was added to the primary antibody (1 μg/mL), and staining was performed as above.
For double staining for GAD/KCC2, the pancreas sections on the glass slides were reacted with solution containing rabbit GAD and guinea pig KCC2 antibodies. For double staining for GAD/insulin, KCC2/glucagon, KCC2/insulin, and KCC2/somatostatin, guinea pig antibody against GAD or KCC2 was mixed with mouse glucagon antibody, rabbit insulin antibody, or rabbit somatostatin antibody. The pancreatic sections were reacted with the mixed antibody solutions and visualized using a mixed solution containing Alexa Flour 488-conjugated anti-guinea pig IgG and Alexa Flour 568-conjugated anti-rabbit or anti-mouse IgG (Life Technologies, Carlsbad, CA, USA), and observed under a laser scanning confocal microscope (FV 1000, Olympus, Tokyo, Japan).
To identify GABA-releasing cells, we performed immunohistochemistry for GAD. Most of the islet cells were GAD-positive (Fig. 1A), as previously demonstrated [13, 42]. There were a few GAD-negative cells at the marginal zone (arrows in Fig. 1A). In the higher magnification photo, cytoplasm was stained with GAD antibody (Fig. 1B, G). Next, we performed immunohistochemistry for KCC2, which underlies the inhibitory action of GABA. KCC2-positive cells were mainly detected at the marginal zone of the islets (Fig. 1D). In the higher magnification photo, KCC2 immunoreactivity was mainly localized near the cell membrane (Fig. 1E, H). This immunoreactivity was completely abolished by adding the peptide antigens used for immunization into the primary antibody solution (Fig. 1C, F), indicating that each antibody specifically bound to GAD or KCC2 in the pancreatic islets. In sections double-stained for GAD and KCC2, no co-labeled cells were detected, indicating that KCC2-positive cells were completely distinct from GAD-positive cells within the pancreatic islet (Fig. 1G–I). Furthermore, GAD-positive cells never contained somatostatin (Supplementary Fig. 1).

Immunohistochemical localization of GAD (A–C, H) and KCC2 (D–G), and double staining for KCC2 and GAD (I) in the pancreatic islets. GAD was localized within pancreatic islets as shown in the lower- (A) and higher-magnification images (B), but a few cells were GAD-negative (arrows in A). The GAD staining disappeared after addition of the GAD peptide used for immunization to generate the primary antibody (+pep in C). KCC2 immunoreactivity was detected at the marginal zone of the islets in the lower-magnification image (D) and near the cell membrane of the endocrine cells in the higher-magnification image (E). The KCC2 staining was also abolished by addition of the KCC2 peptide (+pep in F). GAD-positive cells were KCC2-negative (G–I). Squares in A and D indicate the regions enlarged in B and E, respectively. Bars = (A, C, D, F) 50 μm; (B, E) 25 μm; (G–I) 20 μm.
To characterize the GAD-positive and KCC2-positive cells, we performed double staining for each of these markers and glucagon, insulin, or somatostatin. The GAD-positive cells were insulin-positive (Fig. 2A–C), indicating that the insulin-releasing β cells synthesize GABA, as previously demonstrated [13, 42]. KCC2 immunoreactivity was detected in the glucagon-positive cells (Fig. 2D–F), whereas the KCC2-positive cells were immunoreactive for neither insulin (Fig. 2G–I) nor somatostatin (Fig. 2J–L), suggesting that KCC2 is expressed by α cells, but not by β or δ cells.

Characterization of GAD-positive and KCC2-positive cells. GAD was co-localized with insulin in the central part of the islets (A–C). KCC2-positive cells were localized at the marginal zone (D) and contained glucagon immunoreactivity (D–F). In contrast, KCC2-positive cells at the marginal zone were negative for insulin (G–I) and somatostatin (J–L). Bar = 20 μm.
To reveal the changes in GABAergic signaling in the T1DM islets, we examined the changes in the localization of KCC2 and GAD in the mouse model of STZ-induced DM. Seven days after the first intraperitoneal injection of STZ, body weight was significantly decreased (Fig. 3A), and blood glucose level was markedly increased to 500 mg/dL (Fig. 3B). Compared with normal islets (Fig. 3C), the cytosol of endocrine cells appeared shrunken in the STZ mouse islets (Fig. 3D). Furthermore, lymphatic infiltration was clearly detected (arrows in Fig. 3D). These changes are consistent with previous studies [18, 20, 24], indicating that the T1DM mouse model was successfully produced by STZ injection.

STZ-induced model mice and their pancreatic islets. Body weight was significantly lower in STZ mice compared with control mice (A), and blood glucose level was more than 3-fold higher than in control (B). Compared with control mice (C), the cytoplasm of endocrine cells was shrunken, and lymphatic invasion (arrows) was detected in the STZ mouse islets (D). KCC2-positive cells were also detected at the marginal zone (E), and GAD-positive cells occupied the central zone (F). But immunoreactivity for GAD was decreased in intensity (F). No KCC2/GAD-double positive cells were detected (G). Bars = (C, D) 50 μm; (E–G) 20 μm. **p < 0.01.
Next, we examined the localization of KCC2 and GAD in mice with STZ-induced DM. There were no significant immunohistochemical differences between normal adult (Fig. 1) and control mice (data not shown). KCC2-positive cells did not express GAD (Fig. 3E–G). GAD and insulin were co-localized in the same cells, and these double-positive cells occupied the same central space as those in normal islets. However, the GAD and insulin immunoreactivities were markedly decreased in intensity, and densely-stained cells were markedly reduced in number (Fig. 4A–C). The density of KCC2-postive cells were relatively increased (Fig. 4D, G, J) compared with normal islets. KCC2 was also co-localized with glucagon (Fig. 4D–F), but was not detected in the insulin-positive (Fig. 4G–I) or somatostatin-positive (Fig. 4J–L) cells. These results suggest that in the STZ mouse islets, GAD and insulin immunoreactivities are decreased, whereas KCC2/glucagon-positive cells are increased in density.

KCC2-positive and GAD-positive cells in the STZ mouse islet. GAD was co-localized with insulin, but both immunoreactivities were decreased in intensity (A–C) by STZ injection. KCC2-positive cells contained glucagon (D–F). KCC2 immunoreactivity was not decreased. However, insulin and somatostatin were not detected in the KCC2-positive cells. Bar = 20 μm.
Released GABA binds to the GABAA receptors. When KCC2 is abundantly expressed, the [Cl−]i is low, and GABA induces the influx of Cl− through GABAA receptors. Under these conditions, GABA acts as an inhibitor [2, 30, 31, 44]. In contrast, GABA is excitatory when KCC2 levels are low or near zero. In the pancreatic islets, GABA is concentrated in synaptic-like microvesicles, which are different from insulin-containing vesicles in β cells (Fig. 5) [6, 7, 35]. It is generally accepted that GABAA receptors are expressed in α cells [8, 17, 49, 50]. Our current findings clearly demonstrate that the α cells express KCC2, which is consistent with previous physiological studies showing that GABA is released from β cells, causes membrane hyperpolarization, and reduces glucagon release (Fig. 5) [39, 49, 50]. Here, β cells were found to be KCC2-negative. Many previous studies have shown that β cells express GABAA receptors in rodents [11, 13, 50], suggesting that GABA may mediate membrane depolarization and exert a trophic effect in β cells [8, 32, 41]. In a previous paper, Kursan et al. described that new splice variant of KCC2, lacking exon25, was highly expressed in the β cells using 07-432 antibody [21]. The 07-432 antibody was raised against the common region of all splice variants, including KCC2a, KCC2b, and KCC2a/b S25. They demonstrated that most islet cells, including α, β, and δ cells, were stained with the polyclonal antibody. This result was obviously different from the present result that the β cells were KCC2-negative with our KCC2 antibody. Our antibody was also raised against the common regions of three variants [45]. The results by Kursan et al. [21] should be additionally and carefully re-checked, because of following reasons: (1) In the supplementary figures of the manuscript, immunohistochemical localizations varied among three antibodies, although all antibodies were raised against the same amino acid residues of rat KCC2. Most islet cells were stained by 07-432 and N1/66, whereas only marginal cells, that may be α cells, were labeled by N1/12. The authors did not take account of the difference in KCC2 localization in the islets. Incidentally, the results by N1/12 were same to our present immunohistochemistry in he pancreatic islets (Fig. 1) and Western blotting in our previous study [45]. (2) Qualification of the antibody specificity in the pancreatic islets was insufficient. Absorption test with immunogen was not demonstrated using the pancreatic sections. (3) These findings were not in agreement with previous reports [9, 14, 19, 38, 40, 48] as described in the Introduction. These results suggested that KCC2 may be expressed only in α cells, and the findings of previous studies on GABAergic signaling in pancreatic islets can be explained by the present results. When blood glucose levels are high, GABA and insulin are released from β cells and may cooperate in reducing blood sugar levels.
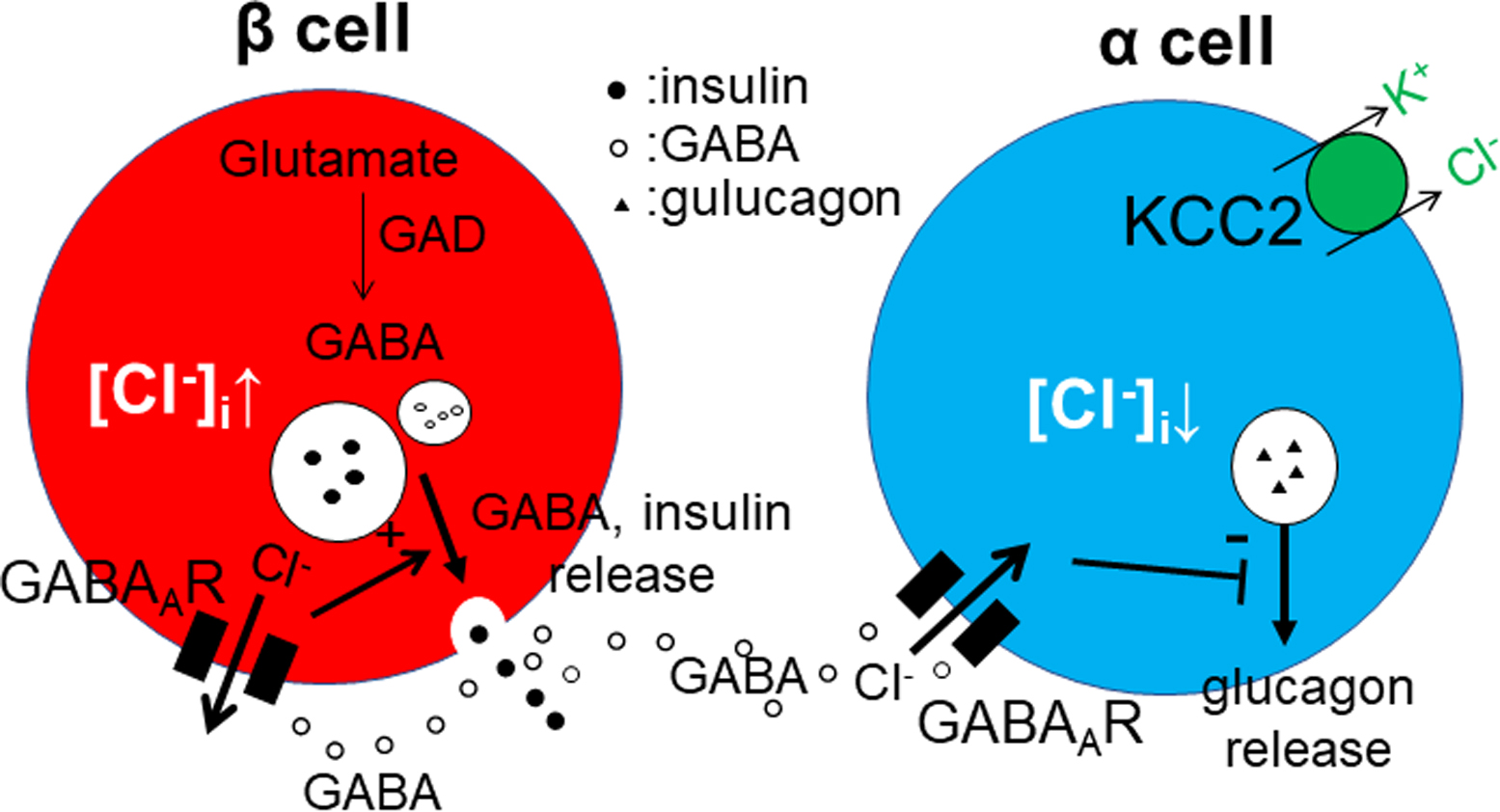
Schematic illustration of GABAergic signaling in the pancreatic islet. GABA is released from β cells and may bind to GABAA receptors on both α and β cells. In α cells, where [Cl−]i is maintained low by KCC2, GABA induces the influx of Cl− and may therefore inhibit glucagon secretion. In contrast, in β cells, where KCC2 is not expressed and [Cl−]i is high, GABA may increase insulin secretion.
In this study, the STZ-injected mice exhibited continuous hyperglycemia and pathological changes in the islets, indicating successful modeling of T1DM in these animals [18, 20, 24]. In their pancreas, islet cells, densely stained with insulin and GAD antibodies, were decreased in number, as in previous reports [41, 47]. In contrast, glucagon-positive cells were increased in density. Previous studies demonstrated the transformation of β cells to α cells and the proliferation of α cells in the STZ mouse [13, 41, 46]. Nevertheless, KCC2 localization was restricted to glucagon-positive cells and was not detected in insulin- or somatostatin-positive cells. KCC2 continued to be expressed in the α cells after proliferation, but disappeared from β cells after transformation. Therefore, the reduction in GABA release in the STZ-injected mice may reduce the inhibition of glucagon release, thereby resulting in an increase in blood sugar. Furthermore, the reduction in GABA levels may also affect the maintenance or regeneration of β cells [4, 23, 33, 34, 47, 48]. Collectively, our current results demonstrate that KCC2 is continuously expressed in α cells, and suggest that GABA may be a good therapeutic target for reducing blood sugar levels and for promoting the regeneration of β cells.
ABC, avidin-biotin-peroxidase complex; Cl−, chloride ion; [Cl−]i, intracellular chloride ion concentration; CNS, central nervous system; D, days after first STD injection; T1DM, type1 diabetes mellites; GABA, gamma-amino butyric acid; GAD, glutamic acid decarboxylase; KCC2, potassium chloride co-transporter 2; PB, phosphate buffer; STZ, streptozotocin
C. S-O. performed all process of this study, including planning, experiments, construction of the figure panels, and writing the text. S. Okada performed the experiment. S. Okamoto and H.M. involved in planning. C.T. wrote the text. The manuscript was checked by all authors.
None.
We are grateful to Makiko Moriyasu-Kurogi Asako Komesu, Akari Katakoka and Natsuki Higa at the Department of Molecular Anatomy for their assistance with preparation of this manuscript. We also thank to Drs. Shiori Kobayashi and Nobuhiko Okura for their valuable discussions. We thank Barry Patel, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
This work was supported by JSPS KAKENHI [Grant-in-Aid for Scientific Research (C) JP18K07823 (CS-O) and 21K06394 (CT)].