2022 Volume 55 Issue 2 Pages 67-73
2022 Volume 55 Issue 2 Pages 67-73
Nodular lymphoid hyperplasia (NLH) of the human colon has been associated with multiple diseases and symptoms. Causes include food allergies, infections, inflammatory bowel disease, and immunodeficiency, and gastrectomy is not usually considered to be the etiology. Nine rats two weeks after total gastrectomy and 12 control rats were sacrificed and submitted for histological examination. In the gastrectomy group, we found lymphoid hyperplasia throughout the entire colon mucosa. The cross-sectional area of lymphoid follicles was increased to be five-fold larger than that in the rats in the control group (sham surgery). Lymphoid follicles were classified into primary and secondary follicles according to the presence/absence of germinal centers; the gastrectomy group had a significantly larger number of secondary follicles. When T cell and B cell classification of lymphocytes was performed, there was no difference between gastrectomy and control groups at T:B = 40:60. When the lymphoid follicles were classified, the proportion of T lymphocytes increased in the secondary follicle (T:B = 40:60) compared with in the primary follicle (T:B = 20:80). Gastrectomy significantly activated lymphocytic intestinal immunity by altering the intestinal environment, causing colonic NLH. Gastrectomy in rats is a good animal model for the study of NLH in colorectal diseases.
Lymphoid follicles are normally present in colonic and intestinal mucosa, particularly among children and young animals. They tend to regress with age, and in older adults they are confined to Peyer’s patches and the appendix in the distal ileum, and in the rectum. Nodular lymphoid hyperplasia (NLH) is the nodular growth of the lymphoid tissue involving the intestinal and colon mucosa, a normal finding of the distal ileum. It is commonly observed in patients with infections and idiopathic inflammatory bowel disease and it requires differentiation from multiple polyposis of the colon and malignant lymphoma. Large nodular lesions require differentiation from malignant mucosal and submucosal tumors, they may also cause intussusception [3, 15]. In many patients, NLH is asymptomatic and can disappear without treatment. It is closely related to food hypersensitivity and inflammatory bowel disease, however, and commonly-presented symptoms include diarrhea, constipation, bloody stools, and abdominal pain. Despite being fairly common, there are no reported laboratory animal models of NLH.
Peyer’s patches of the small intestine are known to develop before birth [2]. Colorectal lymphoid follicles, on the other hand, develop after birth; the intestinal immune system develops with the involvement of intestinal bacteria after birth, and colon lymphoid follicles are thought to develop in relation to intestinal bacteria. Lymphoid follicles are also reported to have differences in function and development between the small and large intestines [2, 7, 14]. Gastrectomy (GX) is a common surgical treatment for gastric cancer and is performed worldwide. It can lead to many postsurgical symptoms, however, including dumping syndrome, eructation and flatulence, anemia, malnutrition, reflux esophagitis, and bone disorders. This study aims to elucidate the etiological relationship between GX and NLH of the human colon.
This study used 10-week-old male Wistar rats. All procedures were approved by our institutional animal care and use committee in accordance with the Principles of Laboratory Animal Care, 1996.
SurgeryGX was performed on nine rats with the removal of the entire stomach and end-to-end anastomosis of the esophagus and duodenum. Sham surgery on 12 rats consisted of manipulation of the viscera only, and these animals were used as controls. Medetomidine hydrochloride (0.15 mg/kg), midazolam (4 mg/kg), and butorphanol tartrate (5 mg/kg) were used to provide adequate sedation, analgesia, and skeletal muscle relaxation. Postoperatively, animals were treated with subcutaneous infusion of Solita-T3 (Ajinomoto, Tokyo, Japan) to prevent dehydration. The rats were given a commercially available powdered diet (MF, Oriental Yeast Co., Ltd., Tokyo, Japan) from the day after surgery. Two weeks after surgery (based on preliminary experiments), tissue samples from the whole colon were collected and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 for two days at 4°C.
Specimen preparation and analysis of mucosal lymphoid folliclesAfter fixation, the whole colon tissue was embedded in paraffin in the longitudinal direction and stained with Hematoxylin-Eosin, then all specimens were observed. A collection of 100 or more lymphocytes was considered to be a lymphoid follicle, and the existence and number of lymphoid follicles in the colon mucosa was measured. Photographs of microscopic images were pasted together to form a composite magnified image (Fig. 1A). The mucosal and submucosal area and lymphoid follicle area were measured using ImageJ software (National Institutes of Health, Bethesda, MD) (Fig. 1B, C). In addition, the lymphoid follicles were classified into primary and secondary follicles according to the presence of the germinal center in the lymphoid follicle (Fig. 1D).
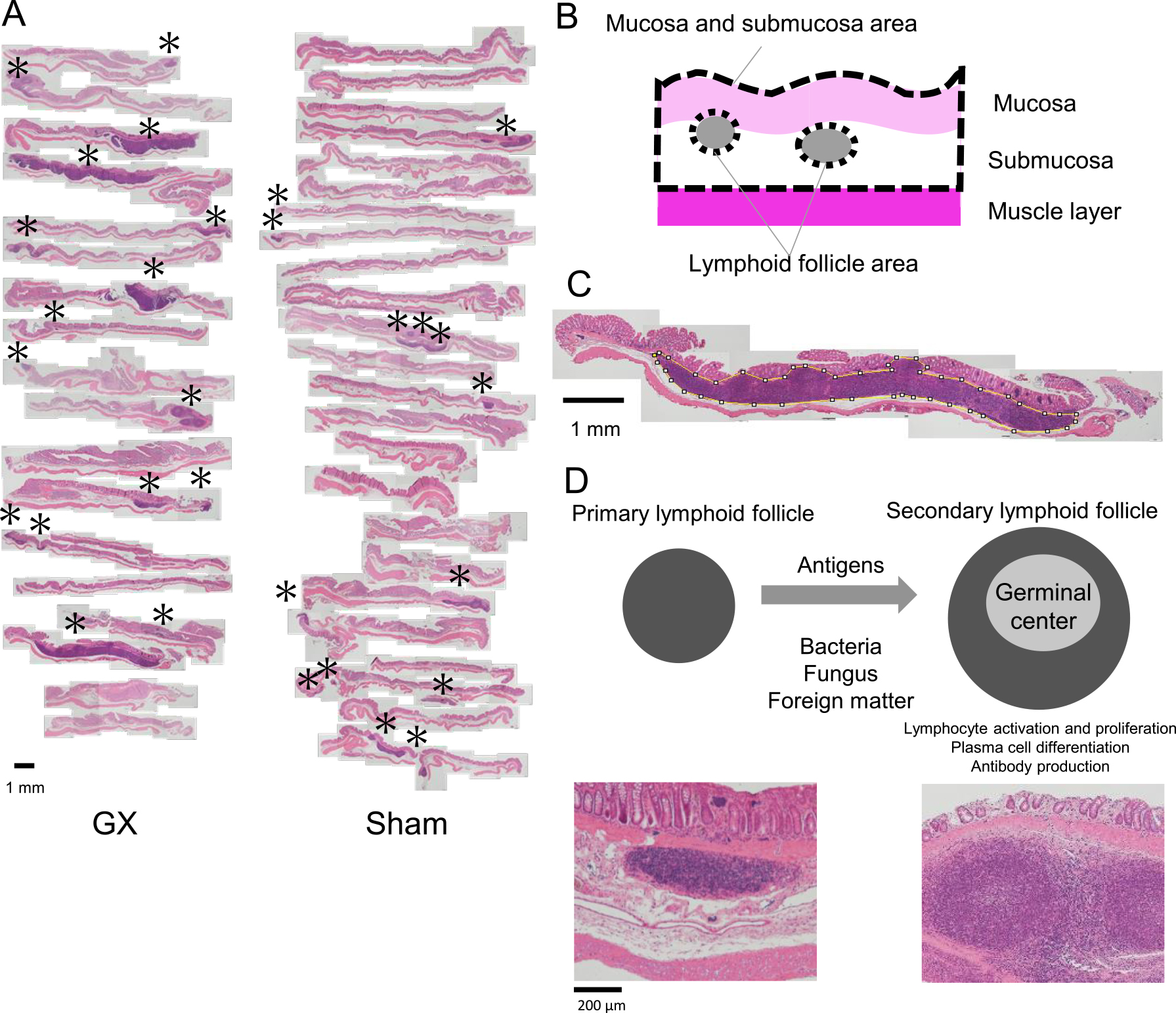
Measured areas of colon specimens. A: Loupe image of colon specimens stained with Hematoxylin-Eosin. B: Mucosa and submucosa area and lymphoid follicle area. C: Lymphoid follicle area measured by ImageJ. D: Classification to primary and secondary lymphoid follicles. * lymphoid follicle.
Additional sections underwent immunohistochemistry staining with anti-CD3 (mouse monoclonal, dilution 1:100, clone F7.2.38, Agilent Dako, Santa Clara, CA), anti-CD79a (mouse monoclonal, dilution 1:50, clone 12E7, Agilent Dako) and anti-CD68 (mouse monoclonal, dilution 1:200, clone KP1, Agilent Dako) antibodies. The antigen activation treatment [8, 9] was performed for CD3 and CD79a section in a pressure cooker for 10 min, 0.01 M EDTA buffer on for CD3, 0.01 M citrate buffer (pH 6) on for CD79a section. On CD68 section, it was treated with proteinase K (20 μg/mL, 15 min) at room temperature. Secondary immunostaining with CSAII (Agilent, Dako) was performed for CD3, and Histofine Simple Stain MAX-PO (MULTI) (Nichirei Biosciences Inc., Tokyo, Japan) for CD79a and CD68. The reaction products were visualized in 20 mg/dl 3,3'-diaminobenzidine tetrahydrochloride solution containing a drop of 30% H2O2. Nuclear counterstaining was by Mayer’s hematoxylin. Measurement of lymphoid follicle positivity was by image analysis software e-HisLym (e-Path Inc. Kanagawa, Japan).
In submucosa, lymphoid follicles were found in 8 of the 9 rats that underwent GX, and 7 of the 12 rats in the control group (P = 0.14) (Table 1). There was an increase in the area of submucosa where lymphoid follicles were present due to the enlargement of the cross section of the lymphoid follicles, some of which had invaded the muscularis mucosae from the submucosa and formed lymphocyte populations in the mucosal layer. Conversely, no lymphoid follicles invaded the muscular layer in the opposite direction (Fig. 1A). No changes, such as inflammation or ulcers, were seen at the epithelium of large intestine mucosa following either GX or sham surgery.
| Lymphoid follicles presence (%) | Mucosa and submucosa area (mm2) | Total lymphoid follicle area (mm2) | Lymphoid follicle area/mucosa and submucosa area (%) | Lymphoid follicle area/lymphoid follicle (mm2) | |
|---|---|---|---|---|---|
| Control (n = 12) | 7 (58.3) | 18.3 ± 5.3 | 0.47 ± 0.56 | 2.3 ± 2.7 | 0.28 ± 0.36 |
| GX (n = 9) | 8 (88.9) | 17.6 ± 1.5 | 2.4 ± 2.2 | 13.8 ± 12.9 | 1.20 ± 1.1 |
| P | NS | NS | 0.01 | 0.01 | 0.02 |
| 0.14 | 0.74 |
The total lymphoid follicle area per section was 2.4 ± 2.2 mm2 following GX, and 0.47 ± 0.56 mm2 following sham surgery, it was five-times larger following GX than after sham surgery (P < 0.05). The submucosal area increased due to the enlargement of the lymphoid follicles, although the mucosal and submucosal area was not different from 17.6 ± 1.5 mm2 following GX and 18.5 ± 5.3 mm2 following sham surgery (P = 0.74). The ratio of lymphoid follicle area to mucosa and submucosa area was 13.8 ± 12.9% after GX and 2.3 ± 2.7% after sham surgery; it was six times larger after GX than after sham surgery, with a significant difference (P < 0.05). The area of lymphoid follicles per lymphoid follicle was 1.20 ± 1.1 mm2 following GX and 0.28 ± 0.36 mm2 following sham surgery; it was more than four times larger following GX than after sham surgery (P < 0.05) (Table 1).
Classification of lymphoid folliclesThe number of lymphoid follicles per section tended to be higher following GX (1.8 ± 0.63) than following sham surgery (1.2 ± 1.1), but without significant difference (P = 0.18). Regarding the classification of lymphoid follicles into primary and secondary follicles, the number of primary follicles was not significantly different between sham (0.92 ± 1.1) and GX (0.67 ± 0.47) groups, but the number of secondary follicles was significantly higher in the GX group than in the sham group: 1.1 ± 0.57 after GX and 0.25 ± 0.43 after sham (P < 0.05) (Fig. 2).

Number of lymphoid follicles by classified lymphoid follicles. * P < 0.05.
CD3-positive cells were regarded as T lymphocytes, CD79a-positive cells were regarded as B lymphocytes, and CD68-positive cells were regarded as macrophages (Fig. 3A). The T and B lymphocyte composition of lymphoid follicles was not significantly different between sham and GX groups (T:B = 40:60, P = 0.91). When lymphoid follicles were divided into primary and secondary follicles, there was no difference between control and GX groups in the primary follicle (T:B = 20:80, P = 0.96), or the secondary follicle (40:60, P = 0.70). The proportion of T lymphocytes was higher in the secondary follicles than in the primary follicles (Fig. 3B).

Immunohistochemistry of lymphoid follicles. A: Immunohistochemistry images of lymphoid follicles. B: Positive rate of lymphocytes by classified lymphoid follicles. C: Positive rate of macrophages by classified lymphoid follicles.
The appearance rate of macrophages in the lymphoid follicles was not significantly different between the two groups: 2.5 ± 2.6% in the control group and 2.7 ± 2.5% in the GX group (P = 0.84). When classified into primary and secondary follicles, the appearance rate tended to be higher in the primary follicles in the GX group than in the control group (GX 3.4 ± 3.9%, sham 1.9 ± 1.3%), but the number of primary follicles was low, the standard deviation was large, and the difference was not significant (P = 0.58) (Fig. 3C).
Mucosa-associated lymphoid tissue is an independent immune system consisting of isolated lymphoid follicles in the mucosa, and is seen in various regions including the gastrointestinal tract, lungs, gonads, eyes, and skin. Lymphocytes carry out immune monitoring against pathogens and foreign substances that cross the epithelial line of defense. Gut-associated lymphoid tissue (GALT) is present in the intestinal mucosa, and a large number of lymphocytes are also found in the lamina propria and intestinal epithelial cells.
In the current study, increased secondary lymphoid follicles with germinal centers were clearly expressed in the submucosa of the colonic epithelium by GX. GX was thought to have made changes in microbial flora and to have created stress to the digestive system. Lymphoid follicles were 6.5 times larger after GX than after sham surgery, most of which were secondary lymphoid follicles with germinal centers. The germinal center is evidence that B cells are activated and proliferated by stimulation of antigen-stimulated T cells, differentiation into plasma cells, and secretion of antibodies, such as promotion of Ig A, resulting in larger lymphoid follicles [2, 3, 5] (Fig. 1D). The presence of large secondary lymphoid follicles suggests that the lymphocytes that encountered the antigen entered the lymphoid follicle and caused a lymphocyte proliferation reaction. No strong inflammatory findings were found in the colonic mucosa, so it is highly possible that there was invasion of harmless antigens (food antigens or resident intestinal bacteria), and there was suppression of the immune response.
Many macrophages in the secondary lymphoid follicle were assumed to phagocytose foreign substances and present antigens to T lymphocytes, but we found no significant difference between those following GX and those following sham surgery. When classified into primary and secondary lymphoid follicles, the appearance rate tended to be higher in the primary follicles following GX, but without significant difference.
The intestinal mucosa is normally in contact with various antigens, and many immunocompetent cells in the intestinal tract are in an activated state. The intestinal mucosa itself is usually not inflamed, meaning the immune response to the majority of harmless antigens (food antigens and resident intestinal bacteria) present in the intestinal tract is suppressed. If there is invasion of harmful antigens into the intestinal tract, quick induction of an immune response is necessary. If for some reason the non-responsiveness to harmless antigens fails and there is a subsequent excessive response, food allergy and inflammatory bowel disease are thought to develop [11, 12].
Gastric resection seems to negatively affect the function of food digestion, inadequately digested products are sent to the small intestine, secretion of gastric acid from the stomach is stopped, and the intestinal environment becomes a higher pH compared to before surgery. Iron reduced by gastric acid is not absorbed by the duodenum and it flows into the large intestine. Secretion of hydrochloric acid and intrinsic factors were thought to be stopped by GX, and there was inhibition of absorption of minerals in the intestinal tract. It is possible that the two-layer mucin barrier that covered the intestinal mucosa may have been modified due to changes in the intestinal environment, making it easier for intestinal bacteria and food antigens to reach the intestinal mucosa. We previously reported that potent gastric acid inhibition by a proton pump inhibitor led to a marked and significant increase in intestinal bacteria [10]. In addition, changes in the intestinal flora before and after GX have been reported [20]. Bacterial invasion from the intestine may have occurred due to changes in the intestinal flora.
Intestinal epithelial cells are exposed to completely different environments on the luminal and basement membrane sides. Intestinal epithelial cells receive various antigen signals from the luminal side, bridge information to immune cells on the basement membrane side, and induce different responses, such as positive immune response or immune tolerance, depending on each antigen. In healthy human intestinal epithelium, there is detection of low expression of Toll-like receptor (TLR)2, which recognizes Gram-positive bacteria such as Bifidobacterium and Lactobacillus, and low expression of TLR4, which mainly recognizes Gram-negative bacteria such as Bacteroides. In many cases of inflammatory bowel disease, there is increased expression of TLR2 and TLR4, resulting in an overresponse to gut microbiota [18, 19]. On the other hand, if the intestinal epithelial cells completely lack stimulation of intestinal bacteria, inflammation may be exacerbated because apoptosis of intestinal epithelial cells increases permeability of the epithelium, increasing the chance of symbiotic bacteria from the epithelium invading the body. Intestinal epithelial cells receiving appropriate levels of “controlled” stimulation from gut bacteria is important. Tightly controlled crosstalk between the intestinal tract and gut microbiota is important for controlling inflammatory response and maintaining homeostasis.
Immune cells play an important role in the mechanism that protects organisms by recognizing, attacking and excluding foreign substances such as bacteria. Innate immune cells activate acquired immunity by digesting pathogens that have invaded the body and transmitting the structural features to the adaptive immune cells, T cells. However, foreign substances, such as bacteria and food-derived antigens, are always present in the intestinal tract, and excessive activation of immune cells in the intestinal mucosa destroys the intestinal tissue and causes enteritis. Immune cells in intestinal mucosa must therefore be in a state of immune tolerance that does not normally respond to foreign substances in the intestine.
Regulatory T cells (Tregs) are acquired immune cells that play an important role in maintaining immune tolerance in the intestine and in suppressing enteritis. Tregs suppress activation of inflammatory T cells that induce inflammation. The proportion of Tregs in CD4+ T cells in lamina propria of the large intestine is reported to be higher than the proportion of Tregs in CD4+ T cells of the small intestine [1]. Many Tregs are present in the lamina propria of the large intestine, and it is expected that the state of remission is likely to be maintained. The large intestine has a huge number of intestinal bacteria compared with the small intestine, the composition of the intestinal flora is different, and the lamina propria is much thicker. The small intestine digests and absorbs nutrients, while the large intestine digests complex molecules and absorbs water and salt. Depending on the site, the intestinal tract has a variety of different functions and barrier mechanisms.
GX disrupts the mucin barrier of the large intestine mucosa, it alters the intestinal flora, leading to the invasion of foreign antigens or intestinal bacteria into the intestinal mucosa and finally immune reaction in GALT. This results in increased growth of isolated lymphoid nodules and secondary lymphoid follicles with formation of germinal centers. The inflammatory reaction that attacks foreign substances was suppressed, however, so no inflammatory findings were observed in the mucosal epithelium.
Colonic NLH after gastrectomy may often occur in humans. However, because the colon is rarely resected and detailed examination of the colon is not performed after gastrectomy, colonic NLH, if it occurs, is likely to go undetected and disappear without treatment in many cases. Meanwhile, the long-term clinical course of colonic lymphoid follicle hyperplasia is not fully understood. Animal experimental studies [4, 6, 13, 16] and a clinical finding [17] indicate that colonic lymphoid nodules are associated with colonic neoplasia. This simple treatment (gastrectomy) causes colonic lymphoid follicle hyperplasia in rats, so this appears to be an excellent animal model not only for studying the pathogenesis, but also the clinical course of this rare condition in adult colonic mucosa. Furthermore, identification of intestinal immune factors is expected to be applied to the development of new therapeutic methods for food allergies and inflammatory bowel diseases.
The authors declare no conflicts of interest associated with this manuscript.
We would like to thank to Dr. Takashi Ueyama for animal experiments, Carl Zeiss Co., Ltd. for analysis of micrographs, and Dr. Shunji Itoh, Dr. Kennichi Kakudo and Dr. Masao Ichinose for helpful discussions. We acknowledge proofreading and editing by Benjamin Phillis.