2023 Volume 56 Issue 6 Pages 95-104
2023 Volume 56 Issue 6 Pages 95-104
Prolonged inactivity in skeletal muscles decreases muscle capillary development because of an imbalance between pro- and antiangiogenic signals, mitochondrial metabolism disorders, and increased oxidative stress. Nucleotides have been shown to exert a dose-dependent effect on disuse-induced muscle atrophy. However, the dose-dependent effect on capillary regression in disused muscles remains unclear. Therefore, this study investigated the dose-dependent effect of nucleotides on capillary regression due to disuse. For this purpose, Wistar rats were divided into five groups as follows: control rats fed nucleotide-free diets (CON), hindlimb-unloaded rats fed nucleotide-free diets (HU), and hindlimb-unloaded rats fed 1.0%, 2.5%, and 5.0% nucleotide diets, (HU + 1.0% NT), (HU + 2.5% NT), and (HU + 5.0% NT), respectively. Unloading increased reactive oxygen species (ROS) production and decreased mitochondrial enzyme activity, thereby decreasing the number of muscle capillaries. In contrast, 5.0% nucleotide-containing diet prevented increases in ROS production and reductions in the expression levels of NAMPT, PGC-1α, and CPT-1b proteins. Moreover, 5.0% nucleotide-containing diet prevented mitochondrial enzyme activity (such as citrate synthase and beta-hydroxy acyl-CoA dehydrogenase activity) via NAMPT or following PGC-1α upregulation, thereby preventing capillary regression. Therefore, 5.0% nucleotide-containing diet is likely to prevent capillary regression by decreasing oxidative stress and increasing mitochondrial metabolism.
Prolonged reduction in loading leads to the regression of muscle capillaries [32]. Further, this regression causes muscle dysfunction, which results in exercise intolerance [25] and insulin resistance [4]. Capillary regression is induced by an imbalance between the vascular endothelial growth factor (VEGF) and thrombospondin-1 (TSP-1), which are pro- and antiangiogenic signals, respectively. Notably, an increase in the number of reactive oxygen species (ROS) is reported to be involved in oxidative stress [32], and a decrease in the oxidative demand because of mitochondrial energy metabolism disorders [33] has been proposed as another mechanism of inactivity-induced capillary regression in skeletal muscles. In particular, a prolonged reduction in skeletal muscle loading reduces the capacity of the muscles to use lipids, leading to decreased metabolic flexibility [27]. According to a previous studies, 2 weeks of hindlimb unloading decreased the mRNA expression levels of citrate synthase (CS) and carnitine palmitoyl transferase I (CPT-1), which are related to fatty acid metabolism in the mitochondria [21]. In addition, the levels of beta-hydroxy acyl-CoA dehydrogenase (β-HAD)—an essential enzyme in fatty acid metabolism specifically involved in the breakdown of fatty acids for energy production—are decreased by approximately 40% with hindlimb unloading for 10 days in the soleus muscles [24].
According to a previous study, intermittent loading during hindlimb unloading does not prevent changes in VEGF and TSP-1, CS activity, and oxidative stress [14]. Another study indicated that muscle contraction using electrical stimulation during hindlimb unloading cannot inhibit the increased inactivity-induced ROS production and decreased succinate dehydrogenase (SDH) activity in mitochondrial metabolism, resulting in an insufficient preventive effect on capillary regression in skeletal muscles [15]. Based on these results, it can be inferred that short-term mechanical stimulation during unloading periods can hardly prevent capillary regression. Accordingly, surrogate intervention strategies are necessary to inhibit an imbalance between angiogenic signals, increase energy metabolism, and decrease oxidative stress to prevent skeletal muscle capillary regression during prolonged unloading.
Nutraceutical strategies have been reported to inhibit an imbalance between angiogenic signals, increase mitochondrial metabolism, and decrease oxidative stress [7, 11, 16, 17]. Nucleotides consist of a nitrogenous base, a pentose sugar, and one or more phosphate groups. They are intracellular compounds that play critical roles in many biological processes [5]. These compounds have antioxidative properties and can decrease oxidative stress [35]. In addition, nucleotides increase nicotinamide adenine dinucleotide (NAD+) production, thereby affecting mitochondrial metabolism in human umbilical vein endothelial cells treated with hydrogen peroxide [37]. Therefore, nucleotides have also been proposed as dietary supplements that can prevent skeletal muscle capillary regression by affecting oxidative stress and mitochondrial metabolism.
However, the effectiveness of these nucleotides in skeletal muscle metabolism may vary depending on the dosage of the supplement. According to a study by Hirayama et al. [14], ingesting nucleotides equivalent to a low-concentration of the dietary amount (approximately 0.7%) during hindlimb unloading does not affect muscle atrophy and capillary regression. However, our previous study indicated that a high-concentration nucleotide-containing diet (approximately 5%) prevents muscle atrophy via the acceleration of satellite cells and activation of the muscle protein synthesis pathway [23]. Thus, it has been reported that low nucleotide concentrations may have little effect on skeletal muscles and their capillaries. Based on these findings, we speculated that dietary ingestion of high concentrations of nucleotides would be necessary for exogenous nucleotides to positively affect skeletal muscle capillaries. Therefore, this study aimed to investigate the dose-dependent effect of nucleotides on disuse-induced muscle capillary regression.
We examined whether low (1.0%)-, medium (2.5%)-, and high (5.0%)-concentration nucleotide ingestion effectively prevents decreased capillary regression by inhibiting decreased angiogenic signals and mitochondrial metabolism during hindlimb unloading. If validated, this study could also prove that nucleotides can prevent both atrophy and capillary regression, making them an exceptional nutritional strategy to address inactivity-induced skeletal muscle regression.
This study was approved by the Institutional Animal Care and Use Committee and conducted in accordance with Kobe University’s Animal Care and Use Protocol (P130930-R2). All experiments were performed according to the National Institutes of Health Guidelines for the care and use of laboratory animals. Thirty 12-week-old female Wistar rats (Japan SLC, Hamamatsu, Japan) were used in this study. All rats were kept in an environmentally controlled animal care facility and allowed to acclimatize for 7 days before the experiments. These rats were individually housed in an isolated and environmentally controlled room at 22°C ± 2°C with a 12-h light–dark cycle.
Experimental diets were designed as nucleotide-free or with supplemental 1.0%, 2.5%, and 5.0% nucleotides via an industrial procedure by Research Diet (New Brunswick, NJ). The compositions of the experimental diets are shown in Table 1. Food intake of the rats was measured daily and averaged over the experimental period (Table 2).
Compositions of experimental diets
| Weight (g%) | Nucleotide-free diets | 1.0% nucleotide diets | 2.5% nucleotide diets | 5.0% nucleotide diets |
|---|---|---|---|---|
| Corn Starch | 51.95 | 50.95 | 49.45 | 46.95 |
| Casein | 20.00 | 20.00 | 20.00 | 20.00 |
| Sucrose | 10.00 | 10.00 | 10.00 | 10.00 |
| Soybean Oil | 7.00 | 7.00 | 7.00 | 7.00 |
| Cellulose | 5.00 | 5.00 | 5.00 | 5.00 |
| Mineral Mix | 3.50 | 3.50 | 3.50 | 3.50 |
| Vitamin Mix | 1.00 | 1.00 | 1.00 | 1.00 |
| α-Corn Starch | 1.00 | 1.00 | 1.00 | 1.00 |
| L-Cystine | 0.30 | 0.30 | 0.30 | 0.30 |
| Synonyms | 0.25 | 0.25 | 0.25 | 0.25 |
| t-butylhydroquinone | 0.00 | 0.00 | 0.00 | 0.00 |
| Nucleotide | — | 1.00 | 2.50 | 5.00 |
Effects of nucleotide-containing diets on physiological parameters
| CON | HU | HU + 1.0% NT | HU + 2.5% NT | HU + 5.0% NT | |
|---|---|---|---|---|---|
| Body mass (g) | 267.8 ± 5.4 | 233.7 ± 4.4* | 227.3 ± 2.3* | 238.2 ± 3.9* | 237.7 ± 4.8* |
| Absolute soleus muscle mass (mg) | 99.4 ± 3.1 | 48.6 ± 1.9* | 48.8 ± 2.0* | 58.8 ± 1.2*†‡ | 57.2 ± 1.4*†‡ |
| Amount of daily diet (g) | 16.7 ± 0.5 | 14.0 ± 0.3* | 13.8 ± 0.5* | 12.5 ± 0.4* | 13.2 ± 0.4* |
| Blood glucose (mg/dl) | 122.0 ± 7.1 | 117.4 ± 6.5 | 118.5 ± 6.7 | 111.9 ± 4.3 | 123.3 ± 10.2 |
| Nonesterified fatty acids (mEq/L) | 0.6 ± 0.05 | 0.6 ± 0.04 | 0.5 ± 0.03 | 0.7 ± 0.04 | 0.6 ± 0.04 |
The average values of body mass, fat mass, absolute soleus muscle mass, blood glucose, and nonesterified fatty acids in the CON, HU, HU + 1.0% NT, HU + 2.5% NT, and HU + 5.0% NT groups are expressed as the mean ± SEM (n = 6 per group).
* p < 0.05 vs. CON. † p < 0.05 vs. HU. ‡ p < 0.05 vs. HU + 1% NT.
The rats were randomly divided into five groups: (1) control rats fed nucleotide-free diets (CON; n = 6), (2) hindlimb-unloaded rats fed nucleotide-free diets (HU; n = 6), (3) hindlimb-unloaded rats fed a diet with supplemental 1.0% nucleotide diets (HU + 1.0% NT; n = 6), (4) hindlimb-unloaded rats fed a diet with supplemental 2.5% nucleotide diets (HU + 2.5% NT; n = 6), and (5) hindlimb-unloaded rats fed a diet with supplemental 5.0% nucleotide diets (HU + 5.0% NT; n = 6). All rats were provided food and water ad libitum.
At the end of the experimental period, the rats were deeply anesthetized via inhalation of 4% isoflurane and killed via intraperitoneal administration of sodium pentobarbital (100 mg/kg). Further, their soleus muscles were quickly removed. The average wet weights of the muscles on both sides were recorded, and then, the soleus muscles were immediately frozen in a dry ice acetone bath and stored at −80°C. Subsequently, blood samples were collected from the inferior vena cava and centrifuged at 3,000 g for 10 min at 4°C. Meanwhile, the muscle and plasma samples were stored at −80°C until histological and biochemical analyses were performed.
Hindlimb unloadingRats in the HU, HU + 1.0% NT, HU + 2.5% NT, and HU + 5.0% NT groups were suspended by their tails according to Morey’s methods [22]. Briefly, the tails of the rats were equipped with a harness, and the rats were suspended by a thread at a height that prevented their hind limbs from pressing against the floor or walls of their cages. Further, the rats’ hindlimbs were unloaded for 2 weeks.
Plasma biochemistryGlucose concentrations in plasma were measured using the Bacillus Calmette-Guerin method and biuret test (Glucose C-II Test Wako; FUJIFILM Wako Chemicals, Tokyo, Japan) [9]. Meanwhile, nonesterified fatty acid (NEFA) levels were measured using the ACS–ACOD method (NEFA C-Test Wako; FUJIFILM Wako Chemicals) [13].
Histological analysesTransverse tissue sections (thickness = 12 μm) were cut from the mid-portion of the right-side muscles of the rats using a cryostat (CM-1510S, Leica Microsystems, Mannheim, Germany) at −25°C and then mounted on glass slides. Further, to observe the capillarity of the soleus muscles, alkaline phosphatase (AP) was used, as described previously [15]. The sections were then briefly fixed with 4% paraformaldehyde and treated with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium for 45 min at 37°C. Using microscopic images, the capillary-to-fiber (C/F) ratio was calculated by counting the capillaries and myofibers in each cryosection. In particular, more than 100 muscle fibers per muscle were counted to calculate the C/F ratio. The NIH ImageJ software (NIH, Bethesda, MD, USA) was used for all measurements.
Dihydroethidium stainingROS production in the soleus muscle was measured using an oxidative fluorescent dihydroethidium (DHE) probe, as described previously [15]. Further, soleus muscles of these rats were thinly sectioned into 12-μm transverse slices using a cryostat microtome. Subsequently, the sections were air-dried, incubated with a 5-μM DHE probe for 30 min at 37°C in a dark box, rinsed with phosphate-buffered saline, and mounted with blue fluorescence 4',6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific, Waltham MA). Notably, when DHE is converted to ethidium bromide, which intercalates into nuclear DNA, red fluorescence is produced. The stained slides were quickly imaged with a fluorescence microscope (BX51; Olympus, Tokyo, Japan) while maintaining the same exposure for every section with appropriate filters for DHE (excitation = 490 nm; emission = 590 nm) and DAPI. Further, the DHE fluorescence intensity was normalized as the ratio of the corresponding DAPI fluorescence intensity [15].
Enzyme activitiesCS and β-HAD activities were measured from the soleus muscles, as described previously [31]. The samples were homogenized in a solution of 10 mM Tris (pH = 7.4), 175 mM KCl, and 2 mM EDTA. Further, measurements were performed using Srere’s method [29]. Briefly, the supernatant was reacted with 5 mM oxaloacetate after adding 100 mM Tris (pH = 7.4), 3 mM acetyl-CoA, and 1 mM 5,5'-dithobis [2-nitrobenzoric acid], and absorbance was measured at 412 nm for 5 min. Notably, the β-HAD activity was measured using Bass’s method [2]. Briefly, the supernatant was reacted with 0.1 mM acetoacetyl-CoA after adding 167 mM triethanolamine (pH = 7.4), 50 mM EDTA, and 0.3 mM NADH, and absorbance was measured at 340 nm for 4 min.
Western blot analysisWestern blot analysis was conducted, as described previously [23]. Briefly, tissue samples were homogenized in an ice-cold homogenizing buffer (Ez RIPA Lysis kit, WSE-7420, ATTO, Tokyo, Japan), and homogenates were centrifuged at 15,000 g for 30 min at 4°C, solubilized in loading buffer (Ez Apply, AE-1430, ATTO), and boiled for 10 min at 80°C. Further, proteins (30 μg/lane) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (e-PAGEL, ATTO) and then transferred to polyvinylidene fluoride membranes. The membranes were blocked for 60 min in 3% bovine serum albumin in Tris-buffered saline with Tween-20 and then incubated with VEGF-A (1:1000; #P49151; Cloud-Clone, Wuhan, China), CPT-1b (1:1000; #22170-1-AP; Protein tech, Rosemont, IL), TSP-1 (1:500; # 18304-1-AP; Protein tech), PGC-1α (1:2000; # 66369-1-Ig; Protein tech), or NAMPT (1:1000; # 11776-1-AP; Protein tech) antibodies overnight at 4°C. Subsequently, the membranes were incubated for 60 min at room temperature with antimouse or antirabbit IgG antibodies conjugated to horseradish peroxidase (1:5000; HAF018/HAF008; R&D systems, Minneapolis, MN). Antibody binding was detected using a chemiluminescent reagent (Ez West Lumi One, WSE-7110, ATTO) and analyzed using an image reader (Lumino Graph I; ATTO). Notably, Ponceau-S (Beacle, Kyoto, Japan) was used as an internal control.
Statistical analysesData are reported as mean ± standard error of the mean. The normality of the data distribution was determined using the Kolmogorov–Smirnov test. One-way analysis of variance was used for the overall group comparisons. Meanwhile, Tukey’s significant difference post hoc test was used to identify differences between individual groups. For all data, p-values of <0.05 were considered statistically significant. All statistical analyses were performed using GraphPad PRISM, version 7.0 (Intuitive Software for Science, California, USA).
Body weight and amount of daily diet of rats that underwent unloading (HU, HU + 1.0% NT, HU + 2.5% NT, and HU + 5.0% NT groups) were significantly lower than those of control rats (CON group; Table 2). All concentrations of nucleotide-containing diets did not affect body weight. Moreover, the absolute soleus muscle mass was significantly lower in rats in the HU, HU + 1.0% NT, HU + 2.5% NT, and HU + 5.0% NT groups than in those in the CON group (p < 0.01, p < 0.01, p < 0.01, and p < 0.01, respectively); however, the total soleus muscle mass was significantly higher in the HU + 2.5% NT and HU + 5.0% NT groups than in the HU group (p < 0.01 and p < 0.01, respectively) and HU + 1.0% NT group (p = 0.01 and p = 0.04, respectively). Nevertheless, no statistically significant differences in blood glucose and NEFA levels were noted among these groups.
Capillary‐to‐fiber ratioRepresentative AP staining patterns in each group are shown in Figure 1A–E. The C/F ratio was lower in the unloading groups (HU, HU + 1.0% NT, HU + 2.5% NT, and HU + 5.0% NT) than in the CON group (p < 0.01, p < 0.01, p < 0.01, and p = 0.01, respectively). This ratio was not statistically significantly different in the HU + 1.0% (p = 0.88) and HU + 2.5% NT (p = 0.38) groups compared with the HU group, but it was significantly higher in the HU + 5.0% NT group than in the HU group (p = 0.03) (Fig. 1F).

Effects of nucleotide-containing diets on the C/F ratio. Representative images of AP staining of the soleus muscle in CON (A), HU (B), HU + 1.0% NT (C), HU + 2.5% NT (D), and HU + 5.0% NT (E) groups. The C/F ratio in each group (F). Bar = 50 μm. Values are expressed as the mean ± SEM (n = 6 per group). * p < 0.05 vs. CON. † p < 0.05 vs. HU.
Representative western blots for VEGF-A and TSP-1 in each group are shown in Figure 2A. There were no statistically significant differences in VEGF-A and TSP-1 protein expression levels among these groups (Fig. 2B and 2C).

Effects of nucleotide-containing diets on VEGF-A and TSP-1 protein expressions. Band images are representative western blots (A), and histograms show quantifications of band densities. The mean levels of VEGF-A (B) and TSP-1 (C) protein expression in the soleus muscles of each group. Values are expressed as the mean ± SEM (n = 6 per group).
Representative DHE staining patterns in each group are shown in Figure 3A–N. The DHE fluorescence intensity was higher in the HU group than in the CON group (p < 0.01). However, the DHE fluorescence intensity was not statistically significantly different in the HU + 1.0% NT, HU + 2.5% NT, and HU + 5.0% NT groups compared with the CON group (p = 0.42, p = 0.24, and p = 0.77, respectively) (Fig. 3O).

Effects of nucleotide-containing diets on ROS production. Representative images of dihydroethidium (A, D, G, J, and L) and DAPI staining (B, E, H, K, and M) and merged images (C, F, I, L, and N) of the soleus muscle in each group. The graph for DHE fluorescence intensity depicts the ratio of DHE and corresponding DAPI fluorescence intensity (O). Bar = 100 μm. Values are expressed as the mean ± SEM (n = 6 per group). * p < 0.05 vs. CON.
CS activities were lower in the HU, HU + 1.0% NT, and HU + 2.5% NT groups than in the CON group (p < 0.01, p < 0.01, and p < 0.01, respectively). However, these activities were not statistically significantly different in the HU + 5.0% NT group compared with the CON group (p = 0.14) (Fig. 4A). Notably, the activity of β-HAD was lower in the HU, HU + 1.0% NT, and HU + 2.5% NT groups than in the CON group (p = 0.02, p = 0.04, and p = 0.02, respectively). However, this activity was higher in the HU + 5.0% NT groups than in the HU, HU + 1.0% NT, and HU + 2.5% NT groups (p = 0.01, p = 0.02, and p = 0.01, respectively) (Fig. 4B).
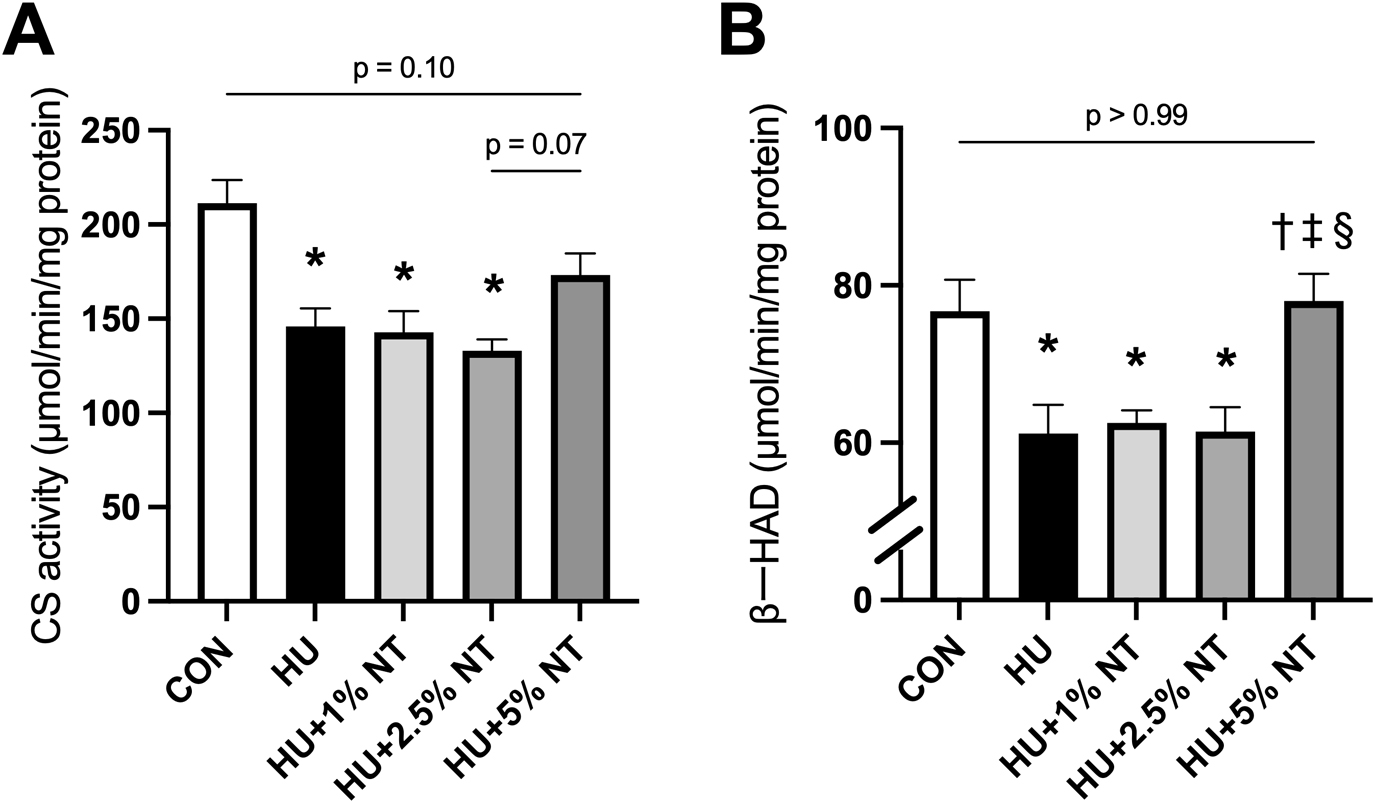
Effects of nucleotide-containing diets on enzyme activities. The histograms for CS and β-HAD activity present the quantification of activity intensity (A, B). Values are expressed as the mean ± SEM (n = 6 per group). * p < 0.05 vs. CON. † p < 0.05 vs. HU. ‡ p < 0.05 vs. HU + 1.0% NT. § p < 0.05 vs. HU + 2.5% NT.
Representative western blots for PGC-1α, CPT-1b, and NAMPT in each group are shown in Figure 5A. The expression levels of PGC-1α were lower in the HU, HU + 1.0% NT, and HU + 2.5% NT groups than in the CON group (p = 0.02, p = 0.03, p = 0.03, respectively). However, these levels were higher in the HU + 5.0% NT group than in the HU, HU + 1.0% NT, and HU + 2.5% NT groups (p < 0.01, p = 0.01, and p = 0.02, respectively) (Fig. 5B). The expression levels of CPT-1b were lower in the HU, HU + 1.0% NT, and HU + 2.5% NT groups than in the CON group (p < 0.01, p = 0.02, and p = 0.02, respectively). However, these levels were higher in the HU + 5.0% NT group than in the HU, HU + 1.0% NT, and HU + 2.5% NT groups (p < 0.01, p < 0.01, and p < 0.01, respectively) (Fig. 5C). The expression levels of NAMPT were lower in the HU group than in the CON group (p < 0.01). However, these levels were higher in the HU + 5.0% NT group than in the HU, HU + 1.0% NT, and HU + 2.5% NT groups (p < 0.01, p = 0.03, and p = 0.03, respectively) (Fig. 5D).

Effects of nucleotide-containing diets on PGC-1α, CPT-1b, and NAMPT protein expressions. Band images are representative western blots (A), and histograms show the quantification of band densities. Mean levels of PGC-1α (B), CPT-1b (C), and NAMPT (D) protein expression in the soleus muscles of each group. Values are expressed as the mean ± SEM (n = 6 per group). * p < 0.05 vs. CON. † p < 0.05 vs. HU. ‡ p < 0.05 vs. HU + 1.0% NT. § p < 0.05 vs. HU + 2.5% NT.
The novel finding of this study is that 5.0% nucleotide-containing diet prevents capillary regression by decreasing oxidative stress and increasing mitochondrial metabolism. However, 5.0% nucleotide-containing diet did not change angiogenic signals in this study. Therefore, we believe that 5.0% nucleotide-containing diet during hindlimb unloading can prevent capillary regression by decreasing oxidative stress and improving mitochondrial metabolism within 2 weeks of unloading.
Two weeks of hindlimb unloading is known to markedly increase oxidative stress and decrease muscle capillaries in the soleus muscle. However, in this study, 2 weeks of hindlimb unloading did not change pro- and antiangiogenic signals, such as VEGF-A and TSP-1 protein expression. Tanaka et al. [32] indicated that the expression of VEGF-A and TSP-1 proteins undergo marked changes within 1 week of hindlimb unloading, but these changes are stable after 2 weeks [32]. In addition, they suggested that oxidative stress is involved in capillary regression within 2 weeks of hindlimb unloading [32]. In the present study, 2 weeks of hindlimb unloading decreased the activity of CS, which is one of the activities of mitochondrial metabolism. Mitochondrial respiratory capacity is affected by the oxygen demand due to physical activity [12]. Meanwhile, ROS production exacerbates mitochondrial respiratory defects after the accumulation of mutations in mtDNA [3]. Previous studies have demonstrated a single relationship between the C/F ratio and CS activity in the soleus muscles of rats [26]. Based on these results, it can be inferred that 2 weeks of hindlimb unloading may induce capillary regression by oxidative stress and mitochondrial metabolism disorders.
Interestingly, in the present study, 1.0% and 2.5% nucleotide-containing diets decreased ROS production, as detected using DHE staining, indicating decreased oxidative stress in the soleus muscles after 2 weeks of hindlimb unloading. This alternation was consistent with the reports by Xu et al. and Zhu et al., which revealed antioxidative effects of nucleotide supplementation [35, 37]. These reports also indicated that ingesting even low-concentration nucleotide diets increases the activity of antioxidant enzymes, such as glutathione peroxidase and superoxide dismutase (SOD), resulting in reduced oxidative stress expressed by malondialdehyde (MDA). Furthermore, Zhu et al. [37] indicated that GMP and CMP were more competent at eliminating ROS and MDA, whereas AMP and UMP were more efficient in enhancing the activity of antioxidant enzymes [37]. In the present study, 1.0% and 2.5% nucleotide-containing diets decreased ROS production. However, both diets did not change the number of capillaries despite reducing ROS production. These results are not consistent with those of a previous study in which antioxidant supplementation prevented capillary regression by ROS production [15, 34]. Differences between the findings of this study and those of the previous study were based on whether there was an effect on mitochondrial metabolism. Xing et al. [34] indicated that chlorogenic acid inhibits capillary regression by increasing the activity of SDH (a mitochondrial rate-limiting enzyme in the tricarboxylic acid cycle) and decreasing the production of ROS during the 2-week hindlimb unloading process. In addition, according to Kanazashi [15], ingesting astaxanthin during 2-week hindlimb unloading period decreases ROS production and increases SDH activity, preventing capillary regression. Alternatively, Hirayama et al. [14] indicated that ingesting only a low-concentration (0.7%) nucleotide-containing diet during hindlimb unloading increases SOD activity but not CS activity, resulting in failure to inhibit capillary regression. Therefore, it is speculated that 1.0% and 2.5% nucleotide-containing diets can inhibit the increase in ROS production but not the decrease in mitochondrial metabolism, resulting in insufficient suppression of capillary regression. Moreover, it has been speculated that the simultaneous prevention of oxidative stress and mitochondrial metabolic dysfunction may be important to prevent hindlimb unloading-induced capillary regression.
Ingestion of a 5.0% nucleotide-containing diet by rats on hindlimb unloading inhibited increases in oxidative stress and mitochondrial dysfunction, thereby preventing capillary regression. In the present study, 5.0% nucleotide-containing diet inhibited ROS generation similar to 1.0% and 2.5% nucleotide-containing diets. Furthermore, 5.0% nucleotide-containing diet increased NAMPT protein expression. It is well known that NAMPT is a rate-limiting enzyme in the salvage pathway that leads to NAD+ biosynthesis [10]. Indeed, NAMPT overexpression increases NAD+ expression in the quadriceps muscles of C57BL/6J mice [6]. Furthermore, the NAMPT knockout mouse model showed reduced NAD+ content, confirming the essential role of NAD+ production [8]. Moreover, a previous study indicated that a nucleotide-containing diet increases NAD+ levels in human umbilical vein endothelial cells treated with hydrogen peroxide [37]. Based on these results, we infer that 5.0% nucleotide-containing diet can increase NAD+ levels by increasing NAMPT expression levels in skeletal muscle.
In the present study, 5.0% nucleotide-containing diet increased PGC-1α protein expression and CS activity. Notably, NAD+ serves as a second messenger in several cellular processes essential for survival and increases PGC-1α function via deacetylation [20]. PGC-1α is a member of a family of transcription coactivators that plays a central role in cellular energy metabolism regulation [18]. In a previous study, PGC-1α overexpression increased CS activity in the tibial anterior muscles of C57BL/6J mice [36]. Thus, 5.0% nucleotide-containing diet is likely to increase CS activity following increased expression of PGC-1α protein by NAMPT protein expression. Other mechanisms have also been proposed for increases in mitochondrial metabolism. Notably, NAD+ is also an essential cofactor involved in the energy production pathways of fatty acid oxidation [28]. Chemical inhibition of NAMPT by FK866 markedly decreases the expression of CPT-1 (a rate-limiting enzyme that transports fatty acids from the cytosol to the mitochondria) in human monocytic cells [19]. Thus, this study assumed that the upregulation of NAMPT by 5.0% nucleotide-containing diet increases CPT-1 protein expression. In addition, a previous study indicated that inhibition of PGC-1α expression decreases CPT-1 protein expression [19]. Furthermore, Suwa et al. reported that the expression level of PGC-1 protein is strongly correlated with β-HAD activity, and these two are critical determinants of mitochondrial fatty acid oxidation in various muscles [30]. Accordingly, 5.0% nucleotide-containing diet increased both CPT-1 protein expression and β-HAD activity via NAMPT or following PGC-1α upregulation. These results indicated that a 5.0% nucleotide-containing diet is likely to prevent capillary regression by decreasing oxidative stress and increasing mitochondrial metabolism.
A limitation of this study is that the mechanism by which 5.0% nucleotide-containing diet markedly increases the mitochondrial metabolic capacity remains unclear. Notably, the dietary nucleotide is absorbed by digestive processes and incorporated into tissue pools, primarily skeletal muscle [5]. Moreover, it has been suggested that nucleotides may promote various intracellular biological mediators by stimulating P2 purinergic receptors [1]. Although it can be expected that 5.0% nucleotide-containing diet had these additive effects, the details have not been clarified. Future studies are warranted to determine the nucleotide pools in skeletal muscles and the activation of P2 purinergic receptors for a detailed mechanism of 5.0% nucleotide-containing diets.
In conclusion, 5.0% nucleotide-containing diet prevented increases in ROS production and reductions in the expression levels of NAMPT, PGC-1α, and CPT-1b proteins in the soleus muscle. In addition, 5.0% nucleotide-containing diet prevented mitochondrial enzyme activity (such as CS and β-HAD activity) via NAMPT or following PGC-1α upregulation, thereby preventing capillary regression. Based on these results, 5.0% nucleotide-containing diet is likely to prevent capillary regression by decreasing oxidative stress and increasing mitochondrial metabolism. Therefore, such diets could be effective for capillary regression by reducing oxidative stress and enhancing mitochondrial metabolism.
The authors have no conflicts of interest to declare.
This study was supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (JSPS KAKENHI; grant numbers: 20K19331, 20KK0227, 22H03453, and 22K19752).
AP: alkaline phosphatase; β-HAD: beta-hydroxy acyl-CoA dehydrogenase; C/F ratio: capillary-to-muscle fiber ratio; CPT-1: carnitine palmitoyl transferase I; CS: Citrate synthase; DAPI: 4',6-diamidino-2-phenylindole; DHE: dihydroethidium; MDA: malondialdehyde; NEFA: Non-esterified fatty acid; PGC-1α: peroxisome proliferating activating receptor-γ coactivator-1 alpha; ROS: reactive oxygen species; SOD: superoxide dismutase; TSP-1: thrombospondin-1; VEGF: Vascular endothelial growth factor