2023 Volume 56 Issue 6 Pages 145-151
2023 Volume 56 Issue 6 Pages 145-151
Osimertinib is a third-generation, irreversible tyrosine kinase inhibitor (TKI) of epidermal growth factor receptor (EGFR) that selectively inhibits both EGFR-TKI-sensitizing and EGFR T790M resistance mutations and has shown efficacy in patients with non-small-cell lung cancer. In this study, we created osimertinib-specific antibodies and developed an immunohistochemistry (IHC) for locating the sites of osimertinib action. Moreover, we located osimertinib–protein conjugates in intestinal, dermal, and lung tissues of rats, thereby using our IHC to visualize the sites of the adverse effects of osimertinib, including diarrhea, skin disorder, and interstitial pneumonia. This report is the first to elucidate the localization of the sites of action of osimertinib in the rat intestine, skin, and lung and is expected to help clarify the mechanism of osimertinib-induced adverse effects.
In recent years, many targeted covalent inhibitors (TCIs) have been developed to selectively form covalent bonds with target proteins [1]. Afatinib, dacomitinib, and osimertinib are representative TCIs that have been approved for non-small cell lung cancer treatment. These drugs irreversibly inhibit epidermal growth factor receptor (EGFR) tyrosine kinase activity by forming covalent bonds with the thiol group of cysteine in the ATP binding pocket via Michael addition. However, Michael acceptor-type TCIs react with various off-target non-specific proteins in a time- and concentration-dependent manner, depending on the drug structure [5]. These highly reactive TCIs have limited clinical applications because they can cause unexpected adverse effects. Previously, we generated specific antibodies against the Michael acceptor-type TCIs afatinib and dacomitinib [14] and successfully developed an immunohistochemical (IHC) assay, which we used to localize covalently bonded drug and protein conjugates in rat intestine and skin [19, 20].
Osimertinib is one of the primary drugs recommended for EGFR gene-positive non-small cell lung cancer [3]. However, it frequently causes adverse effects such as diarrhea and skin disorders, similar to other TCIs [7, 12]. In this study, we created osimertinib-specific antibodies and developed an IHC for localizing the sites of osimertinib action. Moreover, we located osimertinib–protein conjugates in the intestinal, dermal, and lung tissues of rats, thereby using our IHC method to visualize the sites of the adverse effects of osimertinib, including diarrhea, skin disorder, and interstitial pneumonia.
Osimertinib was purchased from Toronto Research Chemicals (Toronto, Canada). Paraformaldehyde and hydrogen peroxide (30% in water) were acquired from Nacalai Tesque (Tokyo, Japan). Histofine Simple Stain MAX-PO (M) was purchased from Nichirei Bioscience (Tokyo, Japan). 3,3,5,5-Tetramethylbenzidine (TMB) was purchased from Boehringer Ingelheim Pharma GmbH (Ingelheim, Germany). 3,3'-Diaminobenzidine tetrahydrochloride (DAB) was purchased from Junsei Chemical (Tokyo, Japan). Bovine serum albumin (BSA) and keyhole limpet hemocyanin (KLH) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). 1-Methylindole and N,N'-dimethylethylenediamine were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). The other reagents and solvents were of the highest grades commercially available.
Preparation of osimertinib–protein conjugatesOsimertinib–protein conjugates were prepared via Michael addition reaction, as shown in Fig. 1. Osimertinib was conjugated with BSA as an immunogen or KLH as a coating antigen. Briefly, a solution of BSA (10 mg, approximately 0.15 μmol) or KLH (5 mg, approximately 95 nM) in 700 μL of 50 mM borax buffer (pH 10.0) was mixed with a solution of osimertinib (5 mg, 10 μmol) in 300 μL of pyridine and incubated at room temperature for 24 hr. The reaction solution was dialyzed with H2O, and the dialyzed conjugate was lyophilized and used as an immunogen or coating antigen. The degree of the coupling of osimertinib to BSA was quantified by using the 2,4,6-trinitrobenzene sulfonic acid (TNBSA) method to determine the primary amine [9]. Approximately 33 osimertinib molecules were coupled to each molecule of BSA, based on the decrease in the primary amine.

Preparation of the immunogen for osimertinib.
Anti-osimertinib serum was prepared according to a previously reported method [10]. Briefly, 5-week-old female BALB/c mice were injected intraperitoneally with 0.1 mg osimertinib–BSA conjugate emulsified with incomplete Freund’s adjuvant. The mice received three injections of the conjugate (0.05 mg) alone at 2-week intervals. Seven days after the last injection, serum was collected from the mice. By applying the indirect non-competitive ELISA method [6], each antiserum sample was tested for the presence of detectable levels of antibodies. Mixed sera from five mice were centrifuged at 1048 × g for 10 min at 4°C and heated for 30 min at 55°C. The obtained antiserum was used directly as an anti-osimertinib antibody for IHC. The experimental protocol was approved by the Sojo University Ethics Review Committee for Animal Experimentation (2021-L-006).
Evaluation of antibody titerWe performed ELISA using a procedure similar to that previously used for anti-bestatin antibody [6]. The wells of microtiter plates were coated with 100 μL of the osimertinib-KLH conjugate (10 μg/mL) or KLH (10 μg/mL) for 60 min at 37°C. The protein-binding sites were then blocked with 1% skimmed milk for 30 min at room temperature. After the addition of 100 μL of serially diluted antibody to each well, the plates were incubated for 90 min at 37°C, followed by the addition of Simple Stain Rat MAX PO (M) (1:1000; 100 μL) and incubated for a further 60 min at 37°C. The wells were washed three times with PBS-BSA and the activity of the enzyme was measured by the addition of 100 μL of 0.42 mM TMB in 0.05 M acetate-citric acid buffer (pH 5.5) containing 0.01% hydrogen peroxide and incubation of the wells for 30 min at 37°C. Then, 100 μL of 2.0 M sulfuric acid was added to each well, and the color intensity was measured using a spectrophotometer at 450 nm with a microplate reader.
Evaluation of antibody specificityAs previously reported and described above [6], the wells of a microtiter plate were coated with the osimertinib-KLH conjugate and blocked with skimmed milk. We added a fixed concentration of anti-osimertinib antibody (1:10,000; 50 μL) to the wells, followed by different concentrations of osimertinib, 1-methylindole or N,N'-dimethylethylenediamine (50 μL). The wells were incubated at 37°C for 2 hr and washed three times with PBS-BSA, followed by incubation with Histofine Simple Stain MAX-PO (M) (1:500 in PBS-BSA) at 37°C for 60 min. The wells were washed three times with PBS-BSA and the activity of the enzyme was measured by the addition of 100 μL of 0.42 mM TMB in 0.05 M acetate-citric acid buffer (pH 5.5) containing 0.01% hydrogen peroxide and incubation of the wells for 30 min at 37°C. Then, 100 μL of 2.0 M sulfuric acid was added to each well, and the color intensity was measured using a spectrophotometer at 450 nm with a microplate reader.
Tissue sample preparationIn this study, we used adult (6–8-week-old) male-specific pathogen-free Wistar rats (Japan SLC, Inc., Shizuoka, Japan) weighing 180–200 g. The experimental protocol was approved by the Sojo University Ethics Review Committee for Animal Experimentation (2021-L-006) and was performed in accordance with standard ethical guidelines for the care and use of laboratory animals [13]. The animals were housed in temperature- and light-controlled rooms (21°C ± 1°C and 12 hr light:12 hr dark) and had free access to standard food and tap water. Three rats were orally administered multiple doses of 10 mg osimertinib/kg body weight for 7 days. No abnormalities were observed in these rats after drug administration. The drug was suspended in methylcellulose (0.5%, m/v water) at a concentration of 10 mg/mL. At 24 hr, the three rats were anesthetized with sodium pentobarbital (60 mg/kg; Abbott Laboratories, North Chicago, IL) and transcardially perfused with 2.5% heparin followed by freshly prepared 4% paraformaldehyde (PFA) in phosphate buffer (pH 7.4). The intestines, skin, and lungs were excised and post-fixed in 4% PFA in phosphate buffer overnight. The tissue was dehydrated with ethanol or xylene and then embedded in paraffin.
IHC staining procedureIHC staining of osimertinib was performed according to our previous methods [19, 20]. Paraffin-embedded intestinal, skin, and lung samples were cut into 5-μm-thick sections. Deparaffinized sections were treated with xylene and ethanol (100%, 95%, 90%, and 70%) and then washed with 10 mM PBS. The sections were treated with 6% H2O2 for 30 min to suppress endogenous peroxidase activity and reduce background staining. The slides were then washed three times with 10 mM PBS for 5 min. Next, 2 N HCl treatment was applied for 30 min to denature the DNA. The slides were washed three times with 10 mM PBS for 5 min before 60 μL blocking buffer (10% normal goat serum [NGS], 1% BSA, and 0.1% saponin in 50 mM Tris-buffered saline [TBS]) was added to the sections and the slides were incubated in a humidified chamber at room temperature for 1 hr (protected from light). Anti-osimertinib serum was diluted 1:1000 with 10% NGS, 0.25% BSA, and 0.1% saponin/50 mM TBS and 60 μL was added dropwise to the slides and reacted in a humidified chamber at 4°C overnight. Absorption controls were treated with 10 μg/mL osimertinib. The next day, the slides were washed three times with 50 mM TBS-0.5% Triton X-100 (TBST) and 50 mM TBS for 5 min. Then, 60 μL of 1:2 diluted Histofine Simple Stain (1% NGS, 1% BSA, and 0.1% saponin/50 mM TBS) was added to the slides and incubated in a humidified chamber at room temperature for 2 hr (protected from light). After the reaction, the slides were washed three times with 50 mM TBST and 50 mM TBS for 5 min. The slides were then immersed in 100 mL DAB substrate (0.05% DAB and 0.012% H2O2 in 50 mM TBS) and incubated at room temperature for 10 min. After color development, they were rinsed several times with distilled water. The slides were then dehydrated through an alcohol series (70%, 90%, 95%, and 100%) and incubated three times in xylene for 5 min each step. Finally, the sections were mounted in Malinol.
The structure of osimertinib comprises an α,β-unsaturated ketone moiety (4-[dimethylamino]-2-buteneamido moiety) that can act as the acceptor molecule for the Michael reaction. The α,β-unsaturated ketone moiety of these drugs forms covalent bonds with electron-rich sections of proteins (e.g., sulfhydryl or amino groups) [18]. Hence, we found that osimertinib antigen bound osimertinib to the carrier protein via a Michael addition reaction. The resulting osimertinib immunogen induced the formation of specific antibodies in each of the five immunized mice.
Evaluation of antibody titer and specificityThe titer of the anti-osimertinib serum produced was evaluated with a dilution ELISA system [6] using osimertinib–KLH conjugate as the solid-phase antigen. Significant binding activity was observed at serial dilutions of anti-osimertinib serum, even at more than 5 × 105 times dilution (Fig. 2).

Evaluation of antibody titer. ELISA measurements of the binding of serially diluted anti-osimertinib serum to the solid phase coated with osimertinib-KLH (closed circles) or KLH (open circles). Each point represents the mean ± SD of three replicates.
The specificity of the anti-osimertinib antibody was determined by testing against the structural components of osimertinib: 1-methylindole and N,N'-dimethylethylenediamine. As shown in Fig. 3, this ELISA was specific to osimertinib and showed no cross-reactivity with 1-methylindole and N,N'-dimethylethylenediamine. The major metabolites of osimertinib in human plasma are AZ5104 and AZ7550 [4]. During long-term therapy, the plasma concentrations of these metabolites are approximately 10% of the plasma concentration of the overall parent exposure [4]. The cross-reactivities of these metabolites could not be confirmed. However, due to the low concentrations of these metabolites, their effect on the analysis of osimertinib in IHC is expected to be minimal.

Evaluation of antibody specificity. Dose-response curve for osimertinib, and cross-reactivity of two partial structures of osimertinib with the anti-osimertinib serum. The curves show the amount (%) of bound enzyme activity (B) for various doses of osimertinib, 1-methylindole, and N,N'-dimethylethylenediamine as a ratio of that bound using the horseradish peroxidase-labeled second antibody alone (B0). ●, Osimertinib; ▲, 1-methylindole; ■, N,N'-dimethylethylenediamine. Each point represents the mean ± SD of three replicates.
Because osimertinib does not possess any amino groups, it is not possible to immobilize it in tissue; however, covalently bonded osimertinib–protein conjugates can be fixed. Therefore, it would be possible to detect the localization of osimertinib–protein conjugates using an IHC with an antibody against osimertinib. In other words, the sites of osimertinib action can be localized.
Immunostaining of the rat intestine (duodenum, jejunum, ileum, and colon), skin, and lung was performed 24 hr after the administration of multiple oral doses of osimertinib (10 mg/kg once daily for 7 consecutive days; Figs. 4–6). Osimertinib was administered at approximately seven times the clinical dose. Multiple doses were administered for 7 days because a single dose did not result in sufficient immunostaining intensity. To verify the specificity of the immunostaining procedure, absorption control experiments were performed in which osimertinib was added to the primary antibody solution at a concentration of 10 μg/mL. These absorption control experiments were all negative (Figs. 4b, 5b, and 6c), demonstrating that the immunostaining was specific for osimertinib. Furthermore, we performed hematoxylin and eosin (H&E) staining on rat intestine, skin, and lung in order to check for histological damage, but none was observed in any of the tissues (data not shown).

Immunohistochemistry for osimertinib–protein conjugates in the rat intestinal tract. (a) Immunostaining was performed with rat intestinal sections (duodenum, jejunum, ileum, and colon) collected 24 hr after multiple oral doses of osimertinib (10 mg/kg once daily for 7 consecutive days). (b) Absorption control experiments were performed in which osimertinib was added to the primary antibody solution at a concentration of 10 μg/mL. arrow: Absorptive epithelial cell, Bar = 50 μm.

Immunohistochemistry for osimertinib–protein conjugates in rat skin. (a) Immunostaining was performed with rat skin collected 24 hr after multiple oral doses of osimertinib (10 mg/kg once daily for 7 consecutive days). (b) Absorption control experiments were performed in which osimertinib was added to the primary antibody solution at a concentration of 10 μg/mL. arrow: Epiderms, arrowhead: Hair follicle, open arrowhead: Sebaceous gland. Bar = 50 μm.
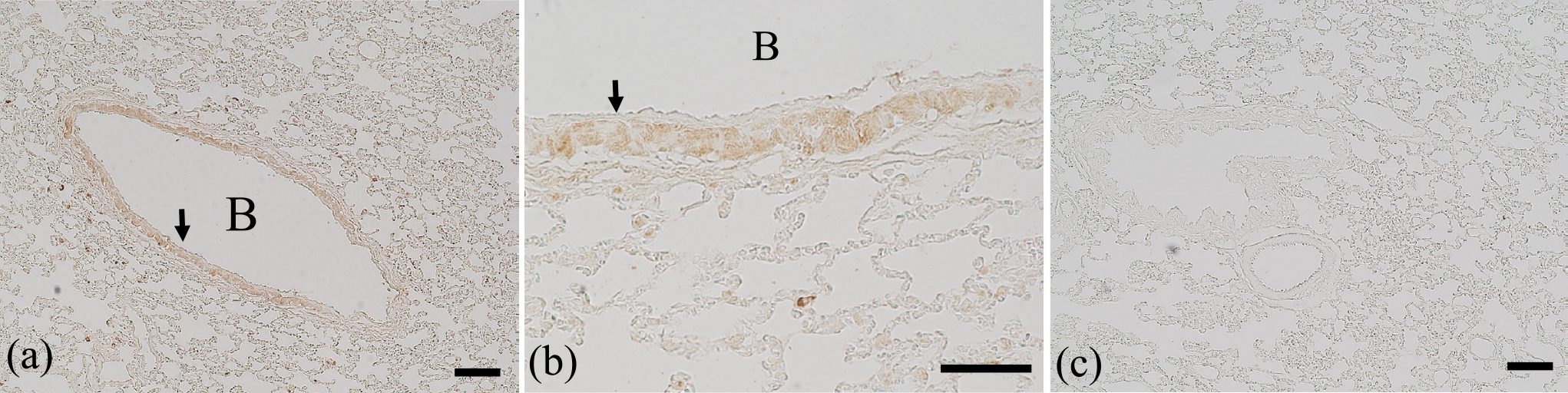
Immunohistochemistry for osimertinib–protein conjugates in rat lung. (a, b) Immunostaining was performed with rat lung collected 24 hr after multiple oral doses of osimertinib (10 mg/kg once daily for 7 consecutive days). (c) Absorption control experiments were performed in which osimertinib was added to the primary antibody solution at a concentration of 10 μg/mL. B: Bronchiole, arrow: Clara cell. Bar = 50 μm.
Immunostaining of the intestines revealed a positive reaction in the jejunum, ileum, and colon, but the duodenum was hardly stained. Most of the ileal tissue was immunopositive, including the absorptive epithelial cells, fibroblasts of the lamina propria mucosa, and the muscularis propria. Given that the expression of EGFR in the intestines is highest in the ileum [17], our results suggest a correlation between intestinal immunopositivity and EGFR expression. In rats, administration of dacomitinib, a second-generation TCI, results in severe ileum injury with elevated monocyte chemoattractant protein 1 (MCP-1) expression and gastrointestinal permeability, and the most notable intestinal histological changes occur in the ileum [17]. Taken together, these findings suggest that osimertinib causes diarrhea as an adverse effect of inhibiting EGFR activity in normal intestinal tissues, especially through damage to the ileum.
In immunostaining of the skin, the epidermis, sebaceous glands, and hair follicles were clearly stained. Moderate positive staining was observed for fibroblasts in the dermis. EGFR in rat skin has been previously detected on the epithelial cells overlying the basement membranes of the epidermis, sebaceous gland, and regions of the hair follicle [8]. The sites with strong immunostaining corresponded almost perfectly to these sites of EGFR expression. These results strongly suggest that osimertinib-induced skin toxicities occur through inhibition of EGFR in the skin by osimertinib.
Immunostaining of the lungs revealed immunopositivity throughout the tissue, with an especially strong reaction observed in the bronchioles. Alveolar epithelial cells generally showed weak immunopositivity. It appeared that the apical domain of the Clara cells was responsible for the strong immunopositivity of the bronchioles. Although alveolar epithelial cells express EGFR, the overall immunopositivity is probably due to osimertinib–EGFR conjugates [2]. However, EGFR expression has not been confirmed in Clara cells, even though they showed strong immunopositivity [16]. Clara cells produce Clara cell secretory protein (CCSP), which suppresses inflammatory cytokine production. CCSP is decreased in the bronchoalveolar lavage fluid in idiopathic interstitial pneumonia [11]. One of the major adverse effects of osimertinib is interstitial pneumonia [7, 15]. Together, these observations indicate the possibility that osimertinib somehow affects Clara cells by causing CCSP to decrease, thereby resulting in interstitial pneumonia. Further studies are needed to determine to which proteins osimertinib binds within Clara cells and how this activity damages the cells.
Although the data are not shown, positive immunostaining reactions were also observed in other rat tissues (liver and kidney). Presumably, osimertinib-protein conjugates are localized in multiple tissues. Michael acceptor-type TCIs react with various off-target non-specific proteins [5]. Therefore, it is necessary to elucidate what kinds of proteins osimertinib reacts within each tissue.
In this study, we created osimertinib-specific antibodies and developed an IHC for locating the sites of osimertinib action. This IHC method for osimertinib could be useful for enabling localization of the sites of osimertinib action. Observation of the localization of sites of osimertinib action is expected to produce important data in studies focusing on the effects and toxicity of osimertinib. This report is the first to elucidate the localization of the sites of osimertinib action in the rat intestine, skin, and lung and is expected to help clarify the mechanism of osimertinib-induced adverse effects.
BSA, bovine serum albumin; DAB, 3,3'-diaminobenzidine tetrahydrochloride; ELISA, enzyme-linked immunosorbent assay; EGFR, epidermal growth factor receptor; IHC, immunohistochemistry; NGS, normal goat serum; PBS, phosphate-buffered saline; TKI, tyrosine kinase inhibitor; TBST, Tris-buffered saline with 0.5% Triton X-100; TBS, Tris-buffered saline; TCI, targeted covalent inhibitor
The authors declare no conflicts of interest.