2024 Volume 57 Issue 4 Pages 131-135
2024 Volume 57 Issue 4 Pages 131-135
Multiple sclerosis, neuromyelitis optica, Guillain–Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy are representative demyelinating diseases of the central and peripheral nervous system. Remyelination by myelin forming cells is important for functional recovery from the neurological deficits caused in the demyelinating diseases. Lysophosphatidylcholine-induced demyelination in mice is commonly used to identify and study the molecular pathways of demyelination and remyelination. However, detection of focally demyelinated lesions is difficult and usually requires sectioning of demyelinated lesions in tissues for microscopic analysis. In this review, we describe the development and application of a novel vital staining method for labeling demyelinated lesions using intraperitoneal injection of neutral red (NR) dye. NR labeling reduces the time and effort required to search for demyelinated lesions in tissues, and facilitates electron microscopic analysis of myelin structures. NR labeling also has the potential to contribute to the elucidation of pathologies in the central and peripheral nervous system and assist with identification of drug candidates that promote remyelination.
Motor, sensory or autonomic dysfunction resulting from abnormalities in the peripheral nerves are collectively known as peripheral neuropathies [12, 14]. Demyelinating diseases such as Guillain–Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy are common causes of peripheral neuropathy [5, 25]. In the peripheral nervous system (PNS), Schwann cell membranes wrap around the axon in layers and form myelin through the synthesis of lipids and myelin proteins [10, 18]; a number of positive and negative regulators of myelin repair have been reported following PNS nerve injury [9, 15, 26]. Peripheral nerves are known to have a higher regenerative capacity than the nerves of the central nervous system (CNS) [9, 15, 26], and promoting regeneration is important to improve the prognosis of demyelinating disease. Understanding the mechanisms underlying Schwann cell remyelination after peripheral nerve injury requires further study, especially over the long durations typically required for regeneration after peripheral neuropathy.
Multiple sclerosis (MS) and neuromyelitis optica are representative demyelinating diseases of the CNS associated with autoimmune abnormalities [4, 8, 11, 17]. Oligodendrocytes, a type of glial cell, form myelin through the extension of many processes and the wrapping of the processes around axons [2, 16]. At present, MS and neuromyelitis optica are difficult to cure entirely. Animal models have therefore often been used in the search for the target pathway and in the evaluation of drug candidates.
The focal demyelination mouse model, wherein neuropathy is induced by injection of lysophosphatidylcholine (LPC) into myelinated nerve fibers, is frequently used to investigate the molecular mechanisms of remyelination, including in studies exploring drug candidates that target the CNS or PNS [3, 7, 19, 20]. However, tissue fixation, sectioning, and histological analysis are required to identify demyelinated lesions in the mouse model for use in molecular and biochemical analyses and evaluation of degeneration and regeneration by electron microscopy (EM).
Recently, we have demonstrated that injection of the supravital stain neutral red (NR) into mice allows macroscopic visualization of demyelinated lesions in freshly dissected CNS and PNS tissue [1, 21, 22]. NR labeling can be used to detect demyelinated lesions in LPC-induced demyelination in the mouse CNS and PNS [22]. In this review, we introduce a NR labeling method that can be used to detect demyelinated lesions in the CNS and PNS by macroscopic observation.
We have recently reported a novel method for macroscopic detection of demyelinated lesions in the CNS and PNS of mice using injection of NR. Mice were injected with 1% LPC into the sciatic nerve to induce injury (Fig. 1A). At 7 days post lesion (dpl), 500 μl of 1% NR in phosphate-buffered saline was administered intraperitoneally; mice were sacrificed 2 hr after NR treatment (Fig. 1A). NR labeling was observed in the LPC-injected sciatic nerve at 7 dpl (Fig. 1B). In contrast, NR was not observed in the contralateral sciatic nerve (Fig. 1B). To confirm demyelination in NR-labeled tissue, we performed immunofluorescence staining for myelin basic protein (MBP; a major constituent of myelin). Demyelination was observed in the LPC-injected sciatic nerve and MBP staining was weak compared with that seen in the contralateral sciatic nerve (Fig. 1C). The NR signal decreased at 14 dpl in the LPC-injected sciatic nerve and had almost disappeared by 21 dpl as a result of remyelination [22]. We have also administered NR dye to mouse models of CNS demyelination, including the experimental autoimmune encephalomyelitis model and focal injection of LPC into the corpus callosum, spinal cord and internal capsule [1, 21]. NR labeling permits rapid identification of demyelinated lesions in CNS and PNS tissues [1, 22]. NR dye also labeled activated microglia, macrophages and reactive astrocytes in the CNS and PNS lesion; this labeling gradually decreased with the resolution of inflammation during remyelination [1, 22]. The cell types that take up NR dye in the lesion are macrophages (iNOS+ M1 type; CD163+ M2 type) and microglia (Iba1+), not injured Schwann cells (MBP+). Incorporated NR colocalized with lysosomal-associated membrane protein type-2-positive lysosomes at 7 and 14 dpl, suggesting that NR is targeted to lysosomes for degradation in the cell [22]. These findings indicate that NR labeling via intraperitoneal injection is a simple method for the macroscopic detection of demyelinated lesions.
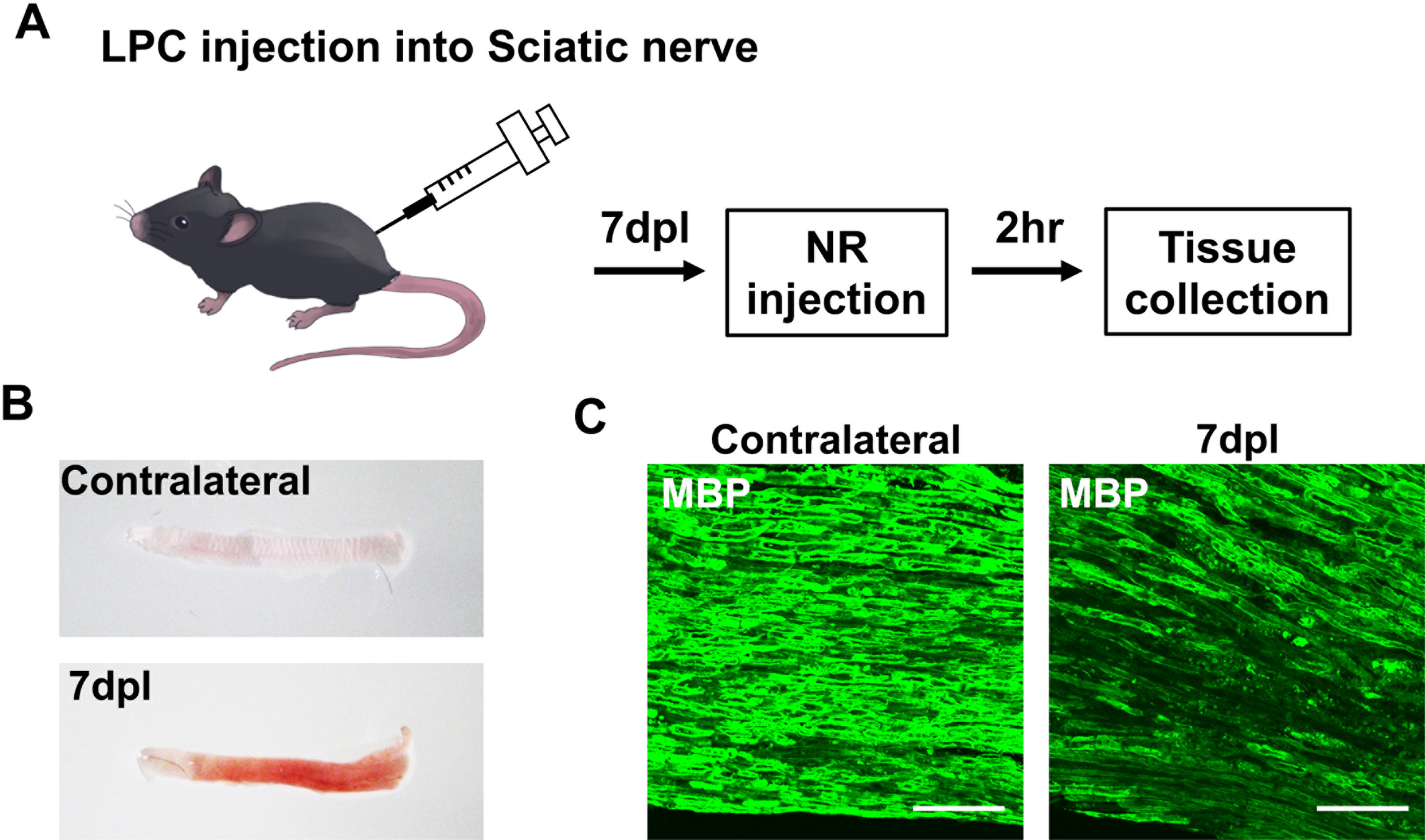
Peripheral nerve lesion identified by neutral red (NR) labeling. (A) Preparation of the sciatic nerve injury model by lysophosphatidylcholine (LPC) injection and NR labeling protocol. NR was injected intraperitoneally 2 hr before sacrifice at 7 days post lesion (dpl). (B) NR-labeled ipsilateral sciatic nerve and unlabeled contralateral sciatic nerve at 7 dpl. (C) Immunofluorescence images of cryosections from LPC-injected mice using anti-myelin basic protein (MBP; green) at 7 dpl and a contralateral sciatic nerve at 7 dpl. Bars = 50 μm.
EM analysis is essential for the evaluation of demyelination and remyelination; therefore, we assessed whether NR labeling can be used alongside EM analysis. Transmission EM (TEM) analysis was performed on NR-labeled sciatic nerves at 7 dpl (Fig. 2A). Lesions labeled with NR were dissected for resin embedding, and semithin and ultrathin sections were prepared (Fig. 2A). Light microscopy analysis of semithin sections stained with toluidine blue (Fig. 2B) and EM analysis (Fig. 2C) indicated that NR-labeled sciatic nerves had demyelinated lesions after LPC injection. Sciatic nerve injury was detected using light microscopy at 7 dpl (Fig. 2B). Thick, compact myelin was observed in contralateral axons using TEM (Fig. 2C). In contrast, myelin debris was found in the lesion labeled by NR at 7 dpl (Fig. 2C, black arrows). Therefore, NR may be incorporated into the lesion by activated microglia and phagocytosing Schwann cells. Demyelinated axons were also observed in the ipsilateral sciatic nerve [22]. Our own research has also shown that NR labeling facilitates identification of demyelinated lesions in mice after CNS injury, and can be used to assess drug efficacy [21]. These results show that NR labeling could be used in combination with EM to reduce the time taken to detect CNS and PNS lesions. These findings suggest that NR labeling could be a useful method to facilitate the evaluation of CNS demyelination, peripheral nerve degeneration and regeneration with EM.

Transmission electron microscopy (TEM) analysis after neutral red (NR) labeling. (A) Experimental design of EM analyses. NR-labeled tissues were dissected at 7 days post lesion (dpl) and embedded in epoxy resin. Semithin sections and ultrathin sections were prepared for analysis by light microscopy and EM. (B) Representative images of semithin sections obtained from lysophosphatidylcholine (LPC)-induced lesions and contralateral sciatic nerve stained with toluidine blue. Sciatic nerve injury was observed in the NR-labeled lesion at 7 dpl. Bars = 20 μm. (C) In the TEM observation, contralateral axons had thick, compact myelin, while myelin debris was observed in the lesion labeled by NR at 7 dpl (black arrows). Bars = 10 μm.
Historically, it has been difficult to detect demyelinated lesions by macroscopic observation; therefore, rapid and accurate examination of lesions required development of a new method to identify focal demyelination. NR dye injection permits clear visualization and rapid detection of demyelinated lesions from CNS and PNS tissues for histological and morphological analysis. NR labeling reduces the time and effort required to find demyelinated lesions in tissues for EM analysis [1, 21], and this method may, therefore, reduce the frequency of errors in lesion detection for ultrastructural analyses of demyelinated tissues [22]. We have recently used NR labeling to evaluate drug efficacy after CNS demyelination [23, 24]. Furthermore, NR labeling has been used to detect demyelinated lesions for transcriptomic and reverse transcription–quantitative polymerase chain reaction analyses in the LPC-induced demyelination mouse model and in a mouse model of neuromyelitis optica spectrum disorder [6, 13]. In future studies, NR labeling could be applied to other pathological models of induced peripheral neuropathy, including the sciatic nerve clash model, the chemotherapy-induced neuropathy model, and the diabetic neuropathy model. NR labeling is currently only used in mice; however, this method could be applied in marmosets, pigs, and other mammals in the future to help further delineate the pathology of demyelinating diseases and assist in developing treatments.
In conclusion, NR labeling is a simple method for detecting demyelinated lesions to assist with the assessment of demyelination and regeneration in CNS and PNS tissues. NR labeling has the potential to be used to elucidate the molecular mechanisms of remyelination and may have utility in the investigation of drug targets or in drug screening for CNS and PNS repair in the future.
The authors declare no conflicts of interest.
We thank Dr. Jeffrey Huang at Georgetown University for research guidance, and members of the Ohno lab, especially Dr. Yasuyuki Osanai and Dr. Tom Kouki, for research support and valuable discussion. This work was supported by a Jichi Medical University Young Investigator Award; the Taiju Life Social Welfare Foundation; the Japan Intractable Diseases (Nanbyo) Research Foundation; the Japan MS Society; the Takeda Science Foundation; the Kobayashi Foundation; the Uehara Memorial Foundation; Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number #20K22690 and #23K14432 to R.Y. and #21H05241 to N.O.. We also thank Jane Bryant, PhD, from Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript.