Abstract
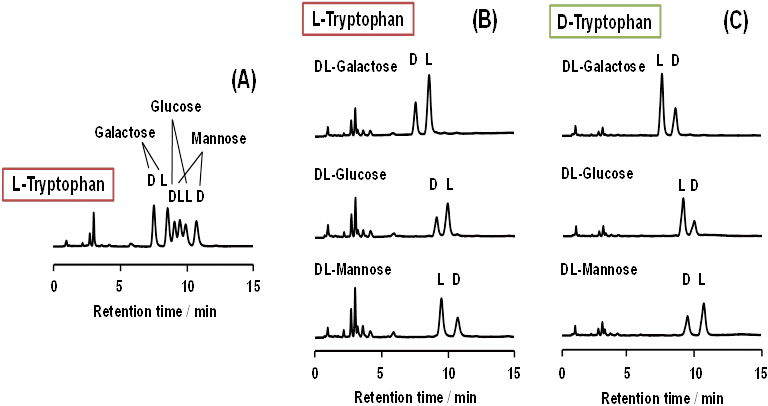
Three reducing monosaccharides (glucose; Glc, galactose; Gal, and mannose; Man) were derivatized with L-tryptophan (L-Trp) under alkaline conditions. The DL-Gal and DL-Man derivatives were chirally resolved by HPLC with an acidic mobile phase, but the DL-Glc derivative was not. All of the three DL-monosaccharide derivatives were simultaneously enantioseparated using HPLC with a SunShell RP-AQUA column (C28) and a basic mobile phase. The optimum mobile phase conditions consisted of 5 mM phosphate and 25 mM tetraborate buffer (pH 9.6) at 20°C. With this system, resolution of D- and L-isomers of the Glc, Gal and Man derivatives were approximately 1.7, 2.2 and 2.4, respectively. When the three monosaccarides were derivatized with L-phenylalanine instead of L-Trp, DL-Gal and DL-Man were enantioseparated under both acidic and basic conditions, but DL-Glc was not. It was observed that enantiomer elution orders of the three monosaccharides derivatized with L-Trp were reasonably reversed when derivatized with D-Trp. It was also revealed that borate anions were required for simultaneous enantioseparation with HPLC.