Abstract
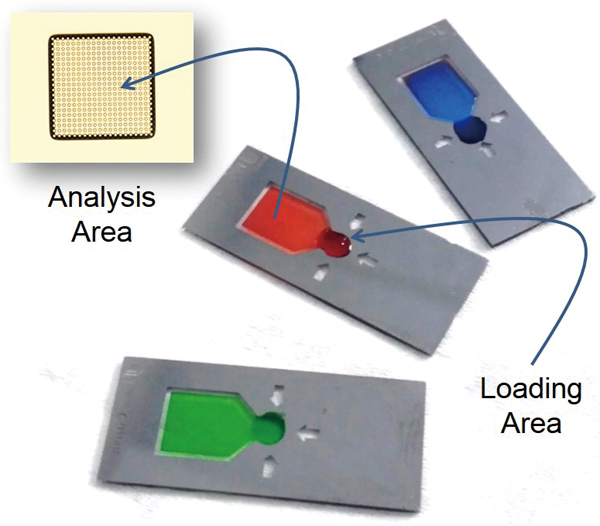
Spectroscopic analysis of solutions containing samples at high concentrations or molar absorptivity can present practical challenges. In absorbance spectroscopy, short optical path lengths or multiple dilution is required to bring the measured absorbance into the range of the Beer’s Law calibration. We have previously reported an open “pillar-cuvette” with a micropillar array that is spontaneously filled with a precise (nL or μL) volume to create the well-defined optical path of, for example, 10 to 20 μm. Evaporation should not be ignored for open cuvettes and, herein, the volume of loaded sample and the rate of evaporation from the cuvette are studied. It was found that the volume of loaded sample (between 1 and 10 μL) had no effect on the Beer’s Law calibration for methyl orange solutions (molar absorptivity of (2.42 ± 0.02)× 104 L mol−1 cm−1) for cuvettes with a 14.2 ± 0.2 μm path length. Evaporation rates of water from methyl orange solutions were between 2 and 5 nL s−1 (30 – 40% relative humidity; 23°C), depending on the sample concentration and ambient conditions. Evaporation could be reduced by placing a cover slip several millimeters above the cuvette. Importantly, the results show that a “drop-and-measure” method (measurement within ∼3 s of cuvette loading) eliminates the need for extrapolation of the absorbance–time data for accurate analysis of samples.