Original Papers
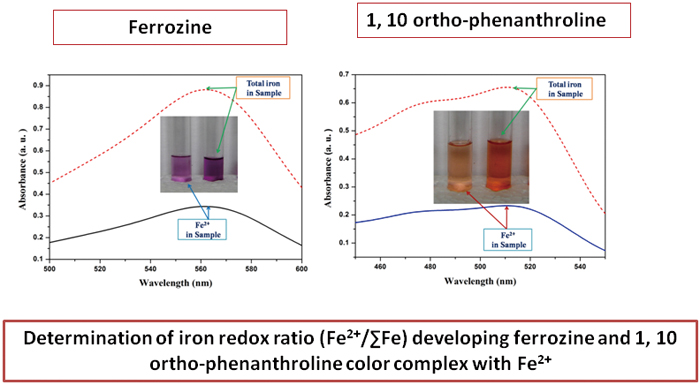
A Comparative Spectrophotometric Study Using Ferrozine and 1,10-Ortho-phenanthroline to Evaluate the Iron Redox Ratio (Fe2+/ΣFe) in Glass Prepared by Microwave Heating
Keywords:
Iron redox ratio in glass,
microwave heating,
barium borosilicate glass,
spectrophotometric method,
ferrozine,
1,10-ortho-phenanthroline
2016 Volume 32 Issue 5 Pages 571-576
Details