Abstract
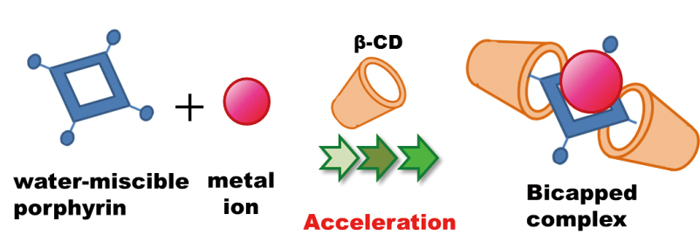
The rate of the complexation reaction between anionic porphyrins and 11 metal ions was found to be accelerated by the presence of β-cyclodextrin (β-CD) in aqueous media at room temperature without the need for additional heating or sonication. The porphyrin complexation reaction with metal ions under aqueous conditions can be difficult due to the strong hydration energy between the metal ions and water. In this study, the specific role of β-CD as an accelerator was determined and found to enhance the typically slow reaction of the porphyrin with metal ions. A significant acceleration effect was exhibited when the model anionic porphyrin, 5,10,15,20-tetraphenyl-21H,23H-porphine-tetrasulfonic acid, and Pb(II) ions were combined in the presence of β-CD. Other than for Hg ion, the addition of β-CD decreased the metalation reaction time from 30 to 2 min. The order in the degree of acceleration was Pb >> Zn, Cd > Cu > Fe, Pd > Sn >> Ag, Co, Mn. Using Pb(II) as the model ion, it was determined that the complexation rate constant was enhanced by a factor of 2.4, while the dissociation rate constant was diminished by a factor of 135 in the presence of added β-CD relative to that in its absence. Overall, the complex was much more stable (formation equilibrium constant 324-fold greater in the β-CD medium. The formation of a ternary complex (cf. bicapped complex; (β-CD)2-porphyrin-metal ion) was demonstrated through the use of nuclear magnetic-resonance spectroscopy and mass spectrometry. This acceleration effect is expected to be applicable systems in which porphyrin ligands are employed for determining of metal ions in chemical analysis and separation science.