2020 Volume 6 Issue 1 Pages 53-60
2020 Volume 6 Issue 1 Pages 53-60
Sarcoidosis, a multi-organ inflammatory condition commonly involving the heart and leading to high morbidity and mortality, is increasingly prevalent. PET imaging with 18F-FDG in conjunction with perfusion imaging is increasingly used for diagnosis, disease characterization, and to guide and follow treatment. However, various challenges remain with regard to protocols, interpretation of image findings, and how best to use test results to guide and monitor therapy. Further investigations of the testing technique, as well as better understanding of disease pathophysiology, are needed for better image utility in order to effectively improve patient outcome.
Sarcoidosis is a worldwide disease manifesting as non-caseating granulomas involving multiple organs, most commonly the lungs, but also the liver, spleen, skin, eyes, central nervous system, bone, parotid gland, and the heart that has recently been recognized as a major cause of morbidity and mortality (1–4). First reported 150 years ago (2), the etiology of sarcoidosis remains unknown, but it is thought to be an immunologic response to unidentified antigen (s) in individuals with a variety of genetically susceptible predispositions, with variability of organ involvement, presentation characteristics and risks. While once considered a rare disease, the reported prevalence is increasing (3), likely in part the result of better diagnostic imaging techniques, and wider recognition of the condition and its high morbidity and mortality (1).
Cardiac involvement had been considered infrequent as it is clinically evident in <10%. However, more recent autopsy and imaging studies report a 20% incidence in the US and >50% in Japan (3), with up to 85% of deaths being cardiac in a Japanese report (5). The presence of isolated cardiac sarcoidosis, which is reported to be approximately 25% (6), is especially problematic in that there may not any sign of the disease before occurrence of an acute life threatening cardiac situation. Clinical features of cardiac sarcoidosis (CS) include conduction abnormalities, left ventricular dysfunction with clinical heart failure (HF), ventricular arrhythmias especially ventricular tachycardia, and tragically sometimes sudden cardiac death (1).
A diagnosis of CS had previously been based on 2006 (revised from 1992) Japanese Ministry of Health and Welfare (JMHW) guidelines (7, 8) that required either cardiac biopsy confirming non-caseating granulomas in someone with a histologic or clinical diagnosis of extra-cardiac sarcoidosis, or histologic or clinical diagnosis of extra-cardiac sarcoidosis with a combination of several major or minor criteria that consist of clinical, ECG, and image findings including gallium-67 citrate (67Ga) and thallium-201 (201Tl) or technetium-99m based perfusion tracer myocardialscintigraphy, as well as delayed enhancement on cardiac magnetic resonance imaging (CMR). These older JMHW criteria (since updated) had required a definitive diagnosis of extracardiac sarcoidosis, and are therefore problematic for diagnosing isolated CS because a traditional endomyocardial biopsy often misses the region with disease. Based on several published studies since then, the recently updated Japanese Circulation Society (JCS) guidelines for a diagnosis of CS (9) add high risk ventricular arrhythmias, abnormal ventricular wall anatomy, high cardiac accumulation of 18F-fluorodeoxyglucose (18F-FDG) and late-gadolinium enhancement (LGE) on CMR to the category of major criteria. In addition, in the absence of histologic evidence of cardiac or extracardiac disease, these guidelines allow the finding of epithelioid granulomas in organs other than the heart. Finally, the JCS guidelines also allow for a diagnosis of isolated CS in the absence of extracardiac disease or histologic cardiac evidence if there is radionuclide imaging evidence, i. e., 67Ga cardiac uptake or high cardiac accumulation of 18F-FDG, and at least three other major cardiac criteria. Alternative criteria are the 2014 Heart Rhythm Society guidelines that also use 18F-FDG and CMR and, while requiring only one “major” criterion, do require a tissue diagnosis of extracardiac disease (10).
For the most part, more established imaging techniques such as chest radiography, ECG and echocardiography, and also biomarker assays such as angiotensin-converting enzyme levels, often provide non-specific findings, and are thus not considered diagnostically reliable (1, 11). CMR and 18F-FDG positron emission tomography (PET) have become the favored diagnostic testing approaches.
CMR is commonly the initial approach, especially important to do before a cardioverter defibrillator or pacemaker is implanted. While there are no specific diagnostic CMR findings, LGE that is patchy or multifocal without involving the endocardial border, especially if the LGE is in basal septal and lateral segments, as well as LGE at right ventricular/left ventricular insertion points that can extend into the RV (“hook sign”), all increase the likelihood of CS, more so if there is no alternative diagnosis (1, 12). Nevertheless, while CMR may indicate high likelihood of CS, LGE does not specifically indicate active inflammation. T2 imaging has potential, but has technical challenges (1). In addition, arrhythmogenic mild CS without significant fibrogranulomatous infiltrative replacement may not be detectable.
Radionuclide imaging with 18F-FDG PET imaging is increasingly undertaken for patients with suspected CS. In the first publication of 18F-FDG PET imaging for CS in 2003, for 17 patients with cardiac sarcoidosis based on histologic evidence of extracardiac disease and fulfillment of prior (1992) JMHW criteria, Yamagishi et al. (13) reported that while few patients had 201Tl perfusion defects or abnormal 67Ga cardiac uptake, 14 patients (82%), who were fasted, had abnormal focally increased (in relation to “normal control” segments) 18F-FDG uptake that was frequently in the basal heart, often with accompanying N-13 ammonia (13NH3) perfusion defects. Two years later, recognizing the need to suppress normal physiologic myocardial 18F-FDG uptake by pre-administering IV heparin, a key study by Ishimaru et al. (14) found pathologic focal, or focal on diffuse, 18F-FDG uptake patterns in 10 (31%) of 32 subjects with known extracardiac sarcoidosis versus no tracer uptake in 30 controls (<0.001). Of patients who met JMHW criteria for cardiac involvement, 100% had focal, or focal on diffuse, tracer uptake, versus 0% having 67Ga uptake and 40% showing 99mTc-sestamibi defects. A 2012 meta-analysis pooling these data with those from 5 other studies established 18F-FDG imaging as an accurate and the best radiotracer technique for diagnosing cardiac involvement by sarcoidosis, with a sensitivity of 89% and a specificity of 78% in reference to 2006 JMHW criteria (15). Based on much work since then, in 2017 consensus documents by the Society of Nuclear Medicine and Molecular Imaging (SNMMI)/American Society of Nuclear Cardiology (ASNC) (3) and by the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine/European Association of Cardiovascular Imaging/ASNC (16) were published describing the role of 18F-FDG PET/CT, in conjunction with perfusion imaging, for evaluating CS. These documents describe recommended dietary preparation to suppress physiologic 18F-FDG myocyte uptake, typical paired metabolic-perfusion PET image patterns likely to indicate ongoing inflammatory disease and/or fibrogranulomatous myocardial infiltration, use of image findings to identify patients at high risk of ventricular tachycardia or death, and potential ways of quantitating the intensity and extent of active disease for risk stratification and following response to therapies. 18F-FDG PET imaging, often in conjunction with CMR imaging of LGE, has become the current standard of care for diagnosing and managing CS (17).
Combined metabolic/perfusion radionuclide image patterns for diagnosis are well described (3, 14, 18). Homogenous perfusion tracer uptake with 18F-FDG limited to blood pool is considered “normal,” while diffuse homogenous 18F-FDG uptake is considered “non-specific” being most likely the result of failure to suppress myocardial 18F-FDG uptake. Normal perfusion with focal 18F-FDG uptake likely represents early disease except if the uptake is limited to the basal lateral wall and nearby papillary muscle that can be a normal variant. A combination of a perfusion defect and focal 18F-FDG uptake in the same place, i.e., a perfusion-metabolic mismatch, or in different areas, indicates advanced disease with both inflammation and fibrogranulomatous scarring. In later disease, there can be large perfusion defects without focal 18F-FDG uptake from previous inflammation having resolved, although this interpretation, often termed “burned out” sarcoidosis, generally requires prior evidence of CS.
Diagnostic utility is increased by assessing extra cardiac regions, with guidelines recommending inclusion of at least the chest, liver, and spleen (3). Such information could help decide the meaning of equivocal cardiac findings, and may show a convenient location, especially a mediastinal node, to obtain a diagnostically definitive biopsy. However, the utility of routine total body imaging is unclear, particularly as this adds time and expense to the procedure.
Findings predicting higher risk of death or ventricular tachycardia include focal perfusion defects or focal 18F-FDG uptake, an approximately 4-fold event increase if both are present, as well as 18F-FDG uptake in the right ventricle which had a 5-fold increase in one study (18). Use of radionuclide imaging to characterize disease severity and help guide therapy is best done through quantitative assessment of 18F-FDG uptake using the standard uptake value (SUV). Although there is no data to support one specific method over another (3), serial assessment of the maximal SUV and the amount of myocardium above various thresholds, i.e., a characterization of the extent and intensity of inflammation, can be used to follow the response to therapy, and improvement in these parameters has been associated with clinical improvement and better LV function (19, 20).
Prior to the recent focus on 18F-FDG PET for diagnosis, SPECT radionuclide imaging was sometimes used. 67Ga is included in diagnostic algorithms and in JSC and HRS guidelines, with the tracer having a high diagnostic specificity. However, 67Ga is now infrequently used because its diagnostic sensitivity is <50%, likely in part the result of poor spatial resolution and difficulty distinguishing cardiac uptake from that in lungs and mediastinum, and because of a high patient radiation exposure of 18.5 mSv from a typical 185 MBq dose (3, 21).
Perfusion SPECT with 201Tl and 99mTc based tracers had previously been used by themselves in an attempt to diagnose sarcoidosis, but image patterns can be confusing as defects can arise from both coronary flow problems and scarring, with defects often not matching coronary territories. Guidelines therefore recommend that given the limited diagnostic accuracy of perfusion imaging and the inability to distinguish scar from active disease, that it be used only together with 18F-FDG PET (3). Nevertheless, a report by Kruse et al. observed that inflammatory sarcoidosis can alter quantitative blood flow measurements on 13NH3 PET perfusion imaging, and that serial following could potentially help assess the effectiveness of therapy (22).
Thus, radionuclide imaging to diagnose, risk stratify and assess response to therapy for CS has demonstrable clinical utility. However, there remain numerous challenges to improving CS radionuclide imaging. Many of the obstacles relate to our limited understanding of sarcoidosis that undoubtedly has multifactorial causes and has differing presentations in individual people. Better understanding of the pathophysiology could help develop personally directed management, with customized imaging approaches including use of existing or new radiotracers, and then individualized therapy, all specifically chosen based on unique disease characteristics in a patient. Improved understanding of the condition should also provide a more reliable reference standard by which to judge the accuracy of imaging techniques as traditional endomyocardial biopsy has a sensitivity <25% (23) (although newer techniques guided by ECG, PET, cardiac MRI, or electroanatomical voltage mapping may improve sensitivity (3)), and JMHW and HRS criteria are suboptimal. It is difficult to effectively use an imaging technique to diagnose and follow a disease if one is not totally sure if disease is present, with some investigators advocating, for the time being, use of a combination of radionuclide imaging, CMR and clinical presentation to estimate disease probability (12).
While the aforementioned issues of understanding sarcoidosis and cardiac involvement are being investigated, more investigatory work is needed on current imaging technique and interpretation of findings. A crucial difficulty with the current protocolis the need to suppress normal physiologic myocardial uptake of 18F-FDG such that tracer is limited to pathologic inflammatory areas. Contemporary protocols call for limiting or entirely eliminating carbohydrate intake for a specific time period followed by fasting prior to the test, with heparin administration recommended by some practitioners prior to tracer administration (3). Osborne et al. (24) reviewed 15 different approaches, indicating a lack of consensus, but a typically recommended diet is high-fat no-carbohydrate food for at least 2 meals, followed by a 4–12 hour fast prior to testing. Other dietary protocols have been proposed including a prolonged (>18 hour) fast (25), an 18 hour fast with a low carbohydrate diet and administration of unfractionated heparin that reduces diffuse LV uptake and increases free fatty acid levels (26) or an even longer (36 hour) low carbohydrate high fat diet followed by an overnight fast (27). One study found that strict, clearly detailed dietary instructions may be important for success (28). Nevertheless, even with strict patient dietary compliance, 10–15% of 18F-FDG sarcoidosis studies are non-diagnostic (17), probably because there can be other confounding factors. For example, proper dietary preparation does not overcome increased 18F-FDG uptake if ischemic or hibernating myocardium is present in a patient who has concomitant coronary disease. Figure 1 shows an example of inadequate myocardial glucose uptake suppression.

56-year-old man with non-ischemic cardiomyopathy (EF = 38%) and episodes of non-sustained ventricular tachycardia who was referred for 13NH3/18F-FDG cardiac sarcoidosis imaging.
a: The initial study showed an enlarged LV with mostly homogeneous 13NH3 perfusion uptake other than small defects of the apex and distal septum (yellow arrows) potentially from sarcoidosis, but with diffuse, largely homogenous uptake on 18F-FDG metabolic images that very much matches the perfusion image pattern.
b: 18F-FDG/CT fusion images confirmed the homogenous metabolic tracer uptake (red arrows) felt likely to be the result of inadequate dietary preparation, thus considered non-diagnostic for active sarcoidosis.
c: A repeat study was performed several months later, again showing perfusion defects of the apex and septum, but now showing that all metabolic tracer was limited to blood pool.
d: Fusion images showed tracer limited to blood pool (red arrows) without myocardial uptake, thus no evidence of active cardiac sarcoidosis.
FDG: fluorodeoxyglucose, CTA: coronary tomographic angiography, HLA: horizontal long axis, SAX: short axis, VLA: verticall ong axis
Of note, several of the preparation protocols cited above recommend, approximately 15 minutes prior to 18F-FDG injection, the intravenous (IV) administration of unfractionated heparin that increases free fatty acid levels and suppresses myocardial and skeletal muscle glucose metabolism (3). However, the extent to which heparin decreases physiologic myocardial uptake to improve image reliability is unclear, with several studies showing mixed benefits (4). Given the uncertain benefit of heparin, with dietary protocol adherence being the most important aspect of patient preparation, it is wise not to administer heparin for any patient already receiving any anticoagulants or for those with known bleeding tendencies. There is also the small likelihood of heparin-induced thrombocytopenia (HIT), and for this reason, the Japanese Society of Nuclear Cardiology recommends against routine administration of heparin (4). The current SNMMI/ASNC guidelines indicate that heparin “can be considered,” (3) while ASNC/SNMMI PET guidelines recommend ensuring that a patient has no contraindications, such as bleeding tendencies, allergy, or history of HIT, prior to administration of IV heparin (29). The matter needs further investigation, and perhaps could be directed only to patients with questionable dietary preparation compliance or when needing to repeat a study after a non-diagnostic one.
An approach that can help interpret images showing potentially inadequate physiologic carbohydrate uptake suppression is to assess 18F-FDG uptake heterogeneity. In the aforementioned study by Ishimaru et al. (14), a heterogeneous appearing “focalon diffuse” pattern was considered consistent with active CS, and others consider this finding likely positive for disease. Nevertheless, careful interpretation is needed when interpreting a focalon diffuse pattern as being diagnostic for sarcoidosis (17), and caution is advised as the pattern may occur under fasting conditions in patients with cardiac dysfunction and heart failure (4). On the other hand, a focal on diffuse pattern with focal 18F-FDG uptake corresponding to perfusion defects, i. e., perfusion-metabolic mismatch, or corresponding to locations with CMR LGE, increases the likelihood of CS, as would be a scenario of complete heart block with proven extra-cardiac sarcoidosis (30).
A more sophisticated assessment of 18F-FDG uptake heterogeneity proposed by Sperry et al. (31) scored FDG tracer uptake using a conventional 17-segment model(32). While the study did not specifically seek diagnostic accuracy, only the coefficient of uptake variation and the summed score in segments with a perfusion-metabolic mismatch were predictive of death, heart transplant, or a ventricular arrhythmia. A more complex computational determination of metabolic tracer uptake heterogeneity, termed “texture analysis” in which information is obtained about relationships among SUV values for adjacent and surrounding pixels or voxels, has been described by Manabe et al (33). In their study, 27 patients with a diagnosis of CS based on Japanese Society of Sarcoidosis and Other Granulomatous disorders guidelines had texture features that significantly and independently distinguished them from 52 oncology patients not known to have CS, with one of the 36 texture features analyzed, i.e., “long-run emphasis,” showing high diagnostic accuracy (AUC=0.91) and high inter-operator reproducibility (intraclass correlation coefficient=0.98).
An additional cause of uncertainty in image interpretation is occurrence of particular patterns that may be normal patient variants, specifically isolated focal 18F-FDG uptake in the lateral wall, particularly in the basal region often with accompanying uptake in the nearby papillary muscle (14, 18). In this situation, the presence of a focal lateral perfusion defect, or other evidence of lateral wall involvement such as focal LGE on CMR or arrhythmic inducibility there on electrophysiologic study has been considered necessary before CS can be confidently diagnosed. Figure 2 shows an example.

A 52-year-old man had transient syncope while stacking fire wood, with emergency services finding a self-terminating wide complex tachycardia. A cardiac MRI showed no abnormalities, but 18F-FDG imaging showed uptake mostly limited to the basal lateral wall (yellow arrows). While there was concern that image findings could be a normal variant, there is also a subtle 13NH3 perfusion defect there (red-arrows), thus a focal perfusion-metabolic mismatch that is likely cardiac sarcoidosis. A subsequent electrophysiologic study showed an inducible ventricular tachycardia that had an anterior/anterolateral/basal LV exit site matching the location of the 18F-FDG uptake.
FDG: fluorodeoxyglucose
As described earlier, CMR is helpful in diagnosing cardiac sarcoidosis, but it is unclear how it should be used in conjunction with 18F-FDG PET. It is difficult to compare the diagnostic accuracies of the techniques as investigative studies are mostly underpowered, there is no satisfactory gold standard for a definitive diagnosis, and the modalities detect different pathologic aspects of the condition–inflammation with PET and scarring with CMR (11). CMR has better resolution and is best for detection and quantification of fibrogranulomatous scarring, while 18F-FDG is currently the better choice for detection of active inflammation and thus often increasing sensitivity in situations where minimal to no scarring has occurred as well as providing a convenient way to effectively guide and follow medical therapy. In terms of diagnostic accuracy, the often cited meta-analysis by Youssef et al. (15) reported a sensitivity of 89% and specificity of 78% with JMHW 2006 criteria used as the reference. For CMR, there are only relatively small observational studies. In one study of 58 patients with pulmonary sarcoidosis, all 12 who had CS by JMHW 2006 criteria had late gadolinium enhancement on CMR, i.e., a sensitivity of 100%, while the specific was 78% (34). In a study comparing 18F-FDG PET to CMR, for 21 patients having known sarcoidosis with suspected cardiac involvement, in reference to 1992 JMHW criteria the sensitivity and specificity of PET were 87.5% and 38.5%, respectively, and for CMR were 75% and 76.9%, respectively (35).
Clearly, there are advantages and disadvantages for each technique. They assess different disease characteristics, and thus in many respects are complementary. A study by Vita et al (12) proposed CMR as the initial test which, if showing no evidence of sarcoidosis, would be the end. If the CMR is abnormal, inconclusive, or if there is high clinical suspicion of disease, PET should be performed.
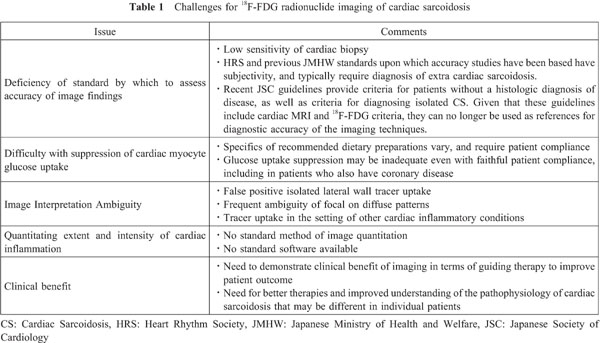
A significant challenge for most clinical nuclear cardiology services, particularly in the US, is that the infrastructure in terms of equipment hardware and software, and of personnel training and experience, has been devoted to myocardial perfusion imaging (MPI) (36). Being a “hot-spot” imaging technique, 18F-FDG imaging for CS requires an interpretive mind-set and analytic software different from that used for MPI. Normalizing metabolic to perfusion images can falsely accentuate mild 18F-FDG uptake. As CS imaging must be able to follow the course of disease, and as visual assessment alone of CS nuclear images is insufficiently sensitive to detect therapeutic response (20), clinicians interpreting studies must be familiar with SUV quantitative techniques. However, methods proposed for using SUV values to assess CS disease severity and follow response to therapy vary among practitioners (3), with no consensus on which, if any, are best. There are no accepted criteria for disease improvement or worsening, nor for the appropriate time intervals of sequential imaging.
A final issue to consider is that other cardiac conditions can produce image patterns similar to sarcoidosis. Among these are myocarditis (3) and Chagas disease (37). Further experience and data accumulation of unique imaging characteristics among these conditions is necessary, requiring better understanding of their pathophysiology and specific findings they may produce.
Cardiac sarcoidosis is a disease with a wide prevalence, and high morbidity and mortality. The goal of performing radionuclide imaging is to noninvasively make an accurate diagnosis that effectively characterizes the extent and severity, and then use imaging to guide patient management and monitor treatment response. The current 18F-FDG PET imaging approach is very good, but the many challenges described must be overcome, with as yet no clear evidence that PET imaging improves patient outcome.
None.
None.
None.