2020 Volume 6 Issue 1 Pages 15-26
2020 Volume 6 Issue 1 Pages 15-26
Background: Obesity increases the risk for development of heart failure (HF) but, when present is likely to be related to better outcomes in patients with HF. This study aimed to clarify the paradoxical prognostic values of visceral obesity in association with cardiac sympathetic function in HF patients.
Methods and Results: A total of 653 consecutive patients with systolic HF who underwent visceral adiposity area (VAA) measurements using a computed tomographic technique were divided into 3 groups: VAA 1, area <80 cm2; VAA 2, area 80–140 cm2; VAA 3, area >140 cm2. Sympathetic innervation was quantified by 123I-MIBG cardiac activity. Patients were followed up for an average of 22 months with a primary endpoint of lethal cardiac events (CE). The CE group (n=200) had a lower late heart-to mediastinum ratio (HMR) and a smaller VAA than those in the non-CE group. Rates of overall CE/HF death were inversely correlated with VAA: 39.2%±6.2% for VAA 1, 27.4%±19.9% for VAA 2 and 24.1%±15.3% for VAA 3. In addition to sudden cardiac death rate, lethal arrhythmic event rate increased in association with visceral fat obesity: 3.0% for VAA 1, 7.5% for VAA 2 and 8.8% for VAA 3. Late HMR identified high-risk sub-populations in each group.
Conclusion: Visceral obesity has paradoxical prognostic implications in terms of HF mortality and lethal arrhythmic/sudden cardiac death events. Cardiac sympathetic denervation and quantitative visceral adiposity are synergistically associated with overall cardiac mortality, contributing to better risk stratification of HF patients.
The global increases in heart failure and obesity have become major public health problems. Obesity is associated with a greater prevalence of cardiovascular risk factors, such as metabolic syndrome, diabetes mellitus, dyslipidemia and hypertension, that may lead to the development of HF (1, 2). On the other hand, there is an inverse relationship between obesity and survival folowing cardiovascular events. In HF patients, malnutrition and low body mass tend to be associated with poor clinical outcomes, whereas HF patients with obesity are likely to have better outcomes. Thus, body fat or obesity itself is likely to have complicated pathophysiological impact, thus creating the obesity paradox, in heart failure outcomes (2–7). The mechanisms behind the dichotomous feature of obesity in heart failure have not been fully elucidated (3). In addition, the association between adiposity and sudden cardiac death is less well understood. The different obesity phenotypes are likely to be responsible for the difference in incidences of atherosclerotic diseases and outcomes in heart failure (4). Rather than an overall index of body fat such as body mass index (BMI), visceral fat obesity has recently been recognized as being more specifically responsible for the development of metabolic syndrome and subsequent atherosclerotic cardiovascular diseases and heart failure (5–7). Sympathetic innervation and function play crucial roles not only in lipid metabolism but also in outcomes in patients with cardiovascular diseases, including heart failure. In contrast to augmented systemic sympathetic drive, sympathetic innervation in the failing heart is impaired. This can lead to lethal cardiac events in HF patients. A number of studies have demonstrated that cardiac 123I-metaiodobenzylguanidine (MIBG) activity evaluated by a neuroimaging technique is an accurate risk biomarker in patients with heart failure independently and synergistically with conventional clinical parameters (8–13). More recent multicenter investigations performed in the US, Europe and Japan (9, 10) have clearly demonstrated the prognostic value of cardiac sympathetic innervation assessed by cardiac 123I-MIBG activity in patients with heart failure. This study was designed to test the paradoxical values of visceral obesity quantified by abdominal computer tomography and cardiac sympathetic innervation assessed by 123I-MIBG activity in chronic HF patients for hard outcome measures.
A total of 653 consecutive patients were prospectively enrolled in this study between April 2011 and March 2017 after a diagnosis of symptomatic congestive heart failure defined as an echocardiographic left ventricular ejection fraction (LVEF) of less than 50%. The patients included 473 males and 180 females with a mean age of 67.3±12.6 years and a mean LVEF of 35.9±10.7%. The diagnosis of heart failure at admission was made by the Framingham criteria (need reference) including typical symptoms (palpitation, dyspnea or orthopnea), neck vein distension, peripheral edema, lung rales, an S3 or S4 gallop, and resting tachycardia. Imaging findings include chest X-ray and two-dimensional echocardiographic examinations of cardiomegaly or left ventricular enlargement, bilateral lung congestion, pleural effusion. Heart failure due to ischemic heart disease was established by the 12-lead electrocardiogram, echocardiography, coronary computed tomography, and nuclear and/or angiographic examinations. Patients who had overt malignancy or hemorrhagic events and patients who had undergone treatment with tricyclic antidepressants, reserpine, guanethidine, phenylpropanolamine and amphetamine, all of which inhibit cardiac 123I-MIBG uptake, were excluded from this study.
Echocardiographic functional assessmentStandard two-dimensional echocardiographic examinations were performed by experienced echocardiographers, who were blinded to detail clinical data, from apical four-, three- and two-chamber views in a left lateral decubitus position using commercially available ultrasound machines equipped with a 2.5-MHz variable frequency transducer. The following echocardiographic data obtained in a stabilized condition before discharge were used for analysis in this study: left atrium diameter (LAD; mm), diastolic left ventricular diameter (LVDd; mm), diastolic ventricular septal wall thickness (IVSTd; mm), diastolic ventricular posterior wall thickness (PTWd; mm), left ventricular ejection fraction (LVEF; %), left ventricular diastolic volume (EDV; ml), left ventricular systolic volume (ESV; ml), E wave velocity (m/sec), left ventricular deceleration time (Dct; msec) and septal E/e' (14).
Assessment of cardiac sympathetic innervation using 123I-MIBGCardiac 123I-MIBG imaging was performed in patients who were in stable condition before discharge using a gamma camera equipped with a low-energy, general-purpose collimator in a fasting and resting condition 15–30 minutes (early image) and four hours (late image) after an intravenous tracer injection of 111 MBq as described previously (10, 13). Cardiac 123I-MIBG activity was measured as the heart-to-mediastinum ratio (HMR) by manually setting a region of interest on the upper mediastinum and whole cardiac regions on a planar anterior image by experienced nuclear medicine technicians without knowledge of any clinical data. 123I-MIBG washout kinetics from the heart were calculated as washout rate (WR) from the early and late cardiac 123I-MIBG activities without a decay correction.
Measurement of visceral adipose tissue areaVisceral adipose tissue area (VAA) was calculated by using a standard computed tomography system (TOSHIBA Aquillion 64 TSX-101A/HA) and the dedicated software, FUJIFILM SYNAPSE VINCENT Ver4.3, (Figure 1) (15, 16).

Abdominal CT and cardiac 123I-MIBG imaging for measurements of VAA and late HMR in two typical cases.
A: Case 1 with LVEF of 28% and late 123I-MIBG HMR of 2.31 had increased VAA of 182.2 cm2 and had no cardiac event during the follow-up period.
B: Despite CRT, Case 2 with LVEF of 31% and late 123I-MIBG HMR of 1.31 had a small VAA of 60.6 cm2 and died from progressive pump failure.
Patients were prospectively and regularly followed up at an outpatient care unit by cardiologists with a primary endpoint of lethal cardiac events consisting of sudden cardiac death, death due to pump failure, lethal ventricular tachyarrhythmias and appropriate implantable cardioverter defibrillator (ICD) shock for at least one year when a patient survived. Overall cardiac events included any of the primary cardiac events, and lethal cardiac events were defined as heart failure and sustained ventricular tachyarrhythmias or ventricular fibrillation that required hospitalization for life-saving by aggressive medical treatment. Patients' outcomes were clarified by reviewing medical records. Sudden cardiac death was defined as witnessed cardiac arrest and death within 1 hour after onset of acute symptoms or unexpected death in patients known to have been well within the previous 24 hours. This study was based on the principles outlined in the Declaration of Helsinki, and informed consent for enrollment in our database and usage for clinical study was obtained according to the guidelines of the ethics committee of our hospital.
Statistical analysisEach statistical value is shown as mean±1 standard deviation (SD). Mean values were compared between the two groups using the unpaired t-test, and categorical variables were compared using the chi-square test. Univariate and multivariate analyses were performed by logistic and Cox hazard models using all variables in Tables 1 and 2. First, when analyzed using an automatic mixed (forward and backward) stepwise method with minimum Baysian information criteria, multivariate model was most appropriate using six variables of NYHA functional class (p<0.0001), amiodarone (p=0.0020), LAD (0.00126), albumin (0.0207), age (p=0.0065) and late 123I-MIBG HMR (p=0.0083). Since we could confirm the agreement between two statistical models for selecting variables, we decided to use Cox hazard analyses for subsequent analysis. Following univariate analysis with all variables, multivariate analysis was performed with statistically significant variables. Receiver operating characteristic (ROC) curve analysis was performed to determine an optimal cutoff value of an independent significant parameter such as HMR of cardiac 123I-MIBG activity. The Kaplan-Meier method was used to create time-dependent, cumulative event-free curves, which were compared using the log-rank test. The computer software program SAS for Windows version 9.4 (SAS Institute, Cary, North Carolina, USA) was used for these analyses. A p-value less than 0.05 was considered significant.


Figure 1 shows abdominal CT and cardiac 123I-MIBG imaging for measurements of VAA and late HMR in two typical cases. Despite the markedly reduced LVEF (28%), Case 1 had preserved late 123I-MIBG HMR (2.31) and increased VAA (182.2 cm2) and had no cardiac events during the follow-up period. Despite CRT, Case 2 with profound reduction of LVEF (33%) and late 123I-MIBG HMR (1.31) had a small VAA (60.6 cm2) and died from progressive pump failure.
During a mean follow-up period of 22±7 months, 200 lethal cardiac events were documented as follows: 152 patients died from pump failure, 17 had lethal ventricular arrhythmias, 20 had sudden cardiac death and 11 had appropriate ICD shocks for lethal ventricular arrhythmias. The appropriate ICD shocks were counted separately from lethal ventricular arrhythmic events.
Patients in the cardiac event group were older and leaner than patients in the non-cardiac event group and they had greater New York Heart Association functional (NYHA) functional class than the non-cardiac event group. A large proportion of patients in the cardiac event group had a history of lethal ventricular tachyarrhythmias, reduced nutritional parameters and eGFR (Table 1). Patients in the cardiac event group also had more systolic dysfunction and less sympathetic innervation than patients in the non-cardiac event group: early 123I-MIBG HMR, 1.63±0.30 vs 1.75±0.27, P<0.0001; late 123I-MIBG HMR, 1.46±0.28 vs 1.63±0.29, P<0.0001 (Table 2). Both intraabdominal and subcutaneous adipose tissue areas in the cardiac event group were significantly smaller than those in the non-cardiac event group (Table 2).
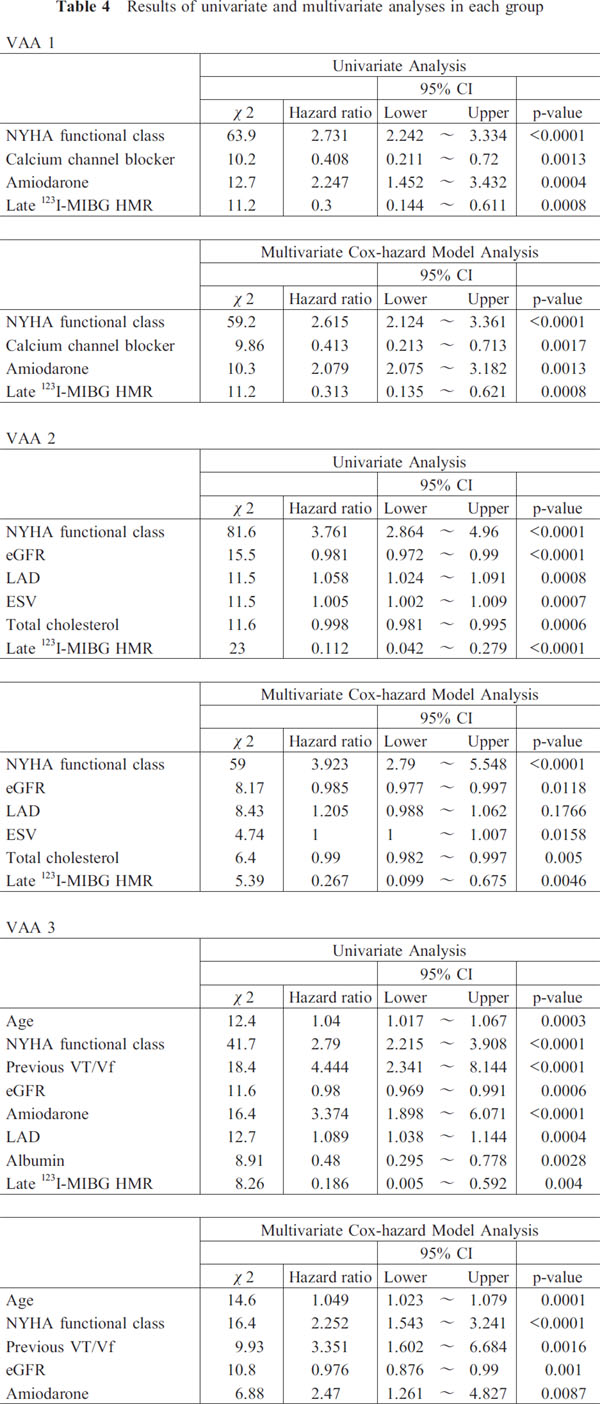
Among the clinical variables, 15 parameters selected by a stepwise analysis were analyzed by Cox multivariate analysis in a stepwise manner (Table 3 and 4). NYHA functional class, amiodarone use and late 123I-MIBG HMR were identified as significant independent prognostic variables with a hazard ratio of 0.850 (CI: 0.782–0.997, P=0.0365) in the overall analysis (Table 3). Based on the grade of VAA, the 653 patients were divided into 3 groups for tertile analysis: VAA 1 (n=232) <80 cm2; VAA 2 (n=226) 80–140 cm2; and VAA 3 (n=195), >140 cm2. Cox multivariate analysis showed that late 123I-MIBG HMR (numerical values) and NYHA functional class were significant independent determinants of lethal cardiac events in each group: hazard ratios of 0.313 (CI: 0.155–0.621, P=0.0008) and 2.615 (CI: 2.124–3.361, P< 0.0001) for VAA 1, 0.267 (CI: 0.099–0.675, P=0.0046) and 3.923 (CI: 2.790–5.548, P<0.0001) for VAA 2, and 0.208 (CI: 0.048–0.836, P=0.0264) and 2.252 (CI: 1.543–3.241, P< 0.0001) for VAA 3, respectively (Table 4). Thus, NYHA functional class and late 123I-MIBG HMR were identified consistently to be significant independent prognostic determinants overall and in each group (VAA1 to VAA3). The data indicated that a lower late 123I-MIBG HMR and a greater NYHA function class were closely related to increased risk of overall cardiac events in any group.

Overall cardiac events and lethal heart failure events were more frequently documented in patients with VAA 1 (39% and 36.2%, respectively) and patients with VAA 2 (27.4% and 19.9%, respectively) than in patients with VAA 3 (24.1% and 15.3%, respectively) (Figure 2). In contrast, lethal arrhythmic event rates, defined as a combination of sudden cardiac death, lethal ventricular arrhythmias and appropriate ICD shocks, increased in parallel with the increase in intraabdominal adipose tissue area: 7 (3.0%) for VAA 1, 17 (7.5%) for VAA 2 and 17 (8.8%) for VAA 3 (Figure 2).

Comparisons of the rates of overall lethal events (closed column), death due to pump failure (open column) and lethal arrhythmic events (slashed column) among 3 groups classified by visceral obesity. Lethal arrhythmic events consisted of sudden cardiac death, lethal ventricular arrhythmias and appropriate ICD shocks against lethal arrhythmias.
Patients with a late 123I-MIBG HMR of less than 1.60, determined by ROC curve analysis, had a significantly lower event-free rate of overall cardiac events than that in patients with late 123I-MIBG of 1.60 or more (Figure 3A). Patients with VAA 1 had a significantly lower event-free rate than did patients in the other two groups, but there was no significant difference in the event-free rate between patients with VAA 2 and patients with VAA 3 (Figure 3B). Figure 4 shows event-free curves when the late 123I-MIBG HMR cut-off value and VAA were combined. When late 123I-MIBG HMR was 1.60 or more, patients with VAA 2 and VAA3 had significantly better outcome curves than did patients with VAA 1 as shown in Figure 4A. Regardless of VAA, late 123I-MIBG HMR of less than 1.60 clearly identified high-risk subpopulations in each group, but VAA 1 patients still had the lowest event-free rate among the three sub-groups (Figure 4B). The late 123I-MIBG HMR clearly discriminated high-risk populations for lethal cardiac events from others using the thresholds determined in each group: 1.45 for group 1, 1.50 for group 2 and 1.63 for group 3 (Figure 5).

Kaplan-Meier event-free curves when patients were classified into two groups by a late 123I-MIBG heart-to-mediastinum ratio (HMR) cutoff value (1.60) (A) and when patients were classified into three groups by visceral adipose tissue area (B).

Kaplan-Meier event-free curves when both the MIBG heart-to-mediastinum ratio (HMR) cutoff value and visceral fat area were combined.
A: Although patients in VAA 2 and VAA3 had better outcomes, patients in VAA 1 patients had significantly lower event free curves even when late 123I-MIBG HMR was 1.60 or more.
B: When late 123I-MIBG HMR was less than 1.60, high-risk subpopulations were clearly identified in each group regardless of the visceral fat area, but patients in VAA 1 patients had the lowest event-free rate among the three sub-groups.

Kaplan-Meier event-free curves of low late 123I-MIBG HMR vs high late 123I-MIBG HMR in the VAA tertiles (right) using each cutoff value of late 123I-MIBG HMR determined by ROC analysis (left).
The findings presented here clarified the paradoxical prognostic implications of visceral adiposity in systolic HF patients; i.e., less visceral fat area is related to increases in overall and heart failure mortality rates but, in contrast, increase visceral adiposity is likely to increased sudden death risk. Assessment of cardiac sympathetic innervation risk-stratified heart failure patients irrespective of but incrementally with adiposity phenotypes identified using a visceral fat area in patients with overt systolic heart failure.
Adipose tissue and sympathetic function in patients with heart failureIn relation to the progression of HF, patients are prone to malnutrition or reduction of visceral fat area by several mechanisms. Systemic and gastrointestinal congestions impair absorption of a sufficient supply of oxygen and energy substrates. Systemically augmented sympathetic tone, which is often observed and known as a classical prognostic biomarker in patients with chronic HF, increases basal energy consumption and accelerates catabolism of lipid and muscular proteins in association with an increased pressure overload and reduced exercise capacity. Recent animal HF models (17–19) have revealed the molecular mechanisms: an increase in noradrenaline concentration in endothelial cells of cardiac and adipose tissues augments lipolysis and then upregulates p53 expression. This, in turn, increases production of active oxygen and inflammatory cytokines. These processes further augment cardiomyocyte injury and dysfunction, creating a vicious cycle of deteriorative reactions in cardiac and adipose tissues. In contrast, reduction of excess sympathetic nerve function or β 2-adrenergic receptor function was shown to improve cardiac function due to reductions in lipolysis, expression of p-53 and subsequent inflammatory reactions (20). Despite a lack of direct evidence of augmented lipolysis and systemic sympathetic tone responsible for decreased visceral adiposity in HF patients, these findings may partially elucidate the interplay between unfavorable cardiac outcomes and reduction in visceral adiposity at an advanced stage of systolic HF.
Visceral obesity and impaired cardiac sympathetic innervationThe results of this study showed the significant prognostic values of impaired cardiac sympathetic innervation assessed by cardiac 123I-MIBG activity (1.60 as a late 123I-MIBG HMR value), which further risk-stratified HF patients at any visceral fat-related cardiac risk. Thus, cardiac sympathetic innervation is independently but synergistically associated with overall cardiac mortality in combination with visceral adiposity. This strongly suggests not only prognostic but also pathophysiological interplays of impaired cardiac sympathetic innervation and decreased visceral adiposity. Previous studies (10–12) showed late 123I-HMR of about 1.6–1.7 to be a threshold for the identification of high-risk HF patients for cardiac death. In addition, cardiac mortality risk increases linearly in relation to a decrease in the late 123I-MIBG HMR value; the lower late 123I-MIBG HMR is, the greater cardiac mortality rate becomes (10). The presented findings basically support the prognostic value of cardiac sympathetic innervation assessed by cardiac 123I-MIBG activity. A synergistic mechanism behind the unfavorable prognostic effects was not determined in this study. Nevertheless, there may be different pathophysiological mechanisms involved in systemic and cardiac conditions responsible for lethal outcomes. Systemic augmentation of peripheral sympathetic tone shown by an elevated plasma norepinephrine (NE) concentration is possibly related to the above-described excess lipolysis-related vicious cycle. On the other hand, increased cardiac NE release is closely related to elevation of MIBG washout kinetics (spill-over), leading to reduced cardiac 123I-MIBG activity in the failing heart and impacting on the progression of heart failure (21).
Visceral obesity and sudden death riskImpairment of cardiac 123I-MIBG activity reflects fundamental pathophysiologic abnormalities in the autonomic nervous seen in HF patients: systemic upregulation of the sympathetic nervous system including alfa-adrenoceptor function in peripheral arteries and, contrarily, downregulation of beta-adrenoceptor function in the failing myocardium. These mechanisms are likely to elucidate the progression of HF and lethal cardiac events. Another HF-related mortality is due to sudden cardiac death. In this context, it is notable that, in contrast to overall cardiac events, lethal arrhythmic events tended to increase in relation to visceral fat area. VAA 3 patients with an increased VAA of more than 140 cm2 had an almost 3-times greater lethal arrhythmic event rate (8.8%) than did VAA 1 patients (3.0%), suggesting a correlation between visceral obesity and sudden death risk. A meta-analysis of results of prospective studies revealed a J-shaped association between adiposity and sudden cardiac death risk (20): lowest risk in the normal weight range and the increased risk with increasing BMI. In large cohort studies using young people, obesity was shown to be closely related to sudden death, particularly when triggered by exercise or the presence of other cardiovascular risk factors leading to coronary artery disease and/or left ventricular hypertrophy (22, 23). Although a paradoxical relationship between obesity (BMI) and mortality risk was also shown in elderly ICD-indicated patients at high risk of sudden death, the major cause of death was heart failure in this population (24). From these findings, in overweight systolic HF patients, excessive physical activity relative to their impaired systemic and/or cardiac functional reserve possibly induces atherosclerotic or ischemic events, leading to lethal arrhythmic events rather than pump failure progression.
Limitations and future perspectivesThe present investigation was designed as a single-center, non-interventional observational cohort study in patients with established systolic heart failure. Whether or not the presented results can be applied to heart failure patients with preserved systolic function remains to be determined. Several metabolic or endocrinological biomarkers possibly related to visceral obesity such as insulin resistance, decreased adiponectin or increased inflammatory cytokines were not clarified in this study. From a health care policy perspective, it may be important to prevent an underweight and overweight conditions in HF patients to reduce the occurrence of lethal cardiac events by an individualized appropriate nutritional intervention. It is also clinically important to determine, using a largerscale population, whether or not and how nutritional modifications can affect clinical outcomes of HF patients with inappropriate viscreral adiposity. Finally, in addition to a nutritional approach, it is necessary to establish how to prospectively select high–risk HF patients who need more aggressive therapeutic interventions, including ICD or CRTD, identified by the presented methods.
ConclusionParadoxical prognostic value of visceral adiposity was clearly demonstrated in this study; a smaller visceral fat area is associated with an increase in overall and heart failure mortality rates, but increased visceral adiposity is related to an increase in sudden death risk. Despite the dichotomous features of visceral adiposity, impairment of cardiac sympathetic innervation is consistently and synergistically associated with increased lethal cardiac events regardless of the grade of visceral adiposity in systolic heart failure patients.
The authors sincerely thank the staff of the Nuclear Medicine Laboratory, Obihiro-Kosei General Hospital, Obihiro, Hokkaido, Japan for cooperation with clinical services and their technical assistance. We also express our gratitude to Dr Ivor Cammack for the editorial support.
None.
There was no conflict of interest to be declared for this study.