2020 年 6 巻 1 号 p. 39-45
2020 年 6 巻 1 号 p. 39-45
Background: Myocardial 18F-fluorodeoxyglucose (18F-FDG) uptake is a sign of active inflammation in patients with cardiac sarcoidosis (CS) under the correct circumstance. However, even under the proper preparation, diffuse myocardial 18F-FDG uptake is frequently observed in the failing heart and misleads the CS disease activity. The aim of this study was to establish the diagnostic value of resting myocardial perfusion single photon emission computed tomography (SPECT) for assessing CS disease activity in patients with diffuse myocardial 18F-FDG uptake.
Methods: We examined 39 patients with either histologically or clinically proven CS. All patients underwent 18F-FDG positron emission tomography (PET) and resting 99mTc-SPECT. The presence of perfusion–metabolic mismatch was evaluated with generating polar maps of 18F-FDG PET and 99mTc-SPECT images.
Results: Increased myocardial 18F-FDG uptake was observed in 33 (85%) of 39 patients. Focal 18F-FDG uptake was detected in 16 patients and diffuse 18F-FDG uptake was seen in 17 patients. Brain natriuretic peptide (BNP) levels were significantly higher in patients with diffuse 18F-FDG uptake than those with focal 18F-FDG uptake (p=0.002). With comparing polar maps of 18F-FDG PET and 99mTc-SPECT images, 8 of 16 patients with diffuse 18F-FDG uptake and myocardial perfusion defects demonstrated perfusion-metabolic mismatch which represented active inflammatory lesions in CS.
Conclusions: Simultaneous evaluation of myocardial 18F-FDG PET and 99mTc-SPECT by polar map analysis provides more relevant information for assessing disease activity in CS than 18F-FDG PET images alone. Perfusion–metabolic mismatch might indicate latent active inflammation in CS patients with diffuse myocardial 18F-FDG uptake, who had advanced heart failure.
Sarcoidosis is a systemic inflammatory disorder characterized by non-necrotizing granulomas and concomitant fibrosis. Cardiac involvement, called cardiac sarcoidosis (CS), is found in nearly 60% of Japanese patients with systemic sarcoidosis (1) and associated with a high risk of morbidity and mortality. In 2016, the Japnese Circulation Society (JCS) published guidelines for the diagnosis and treatment of CS (2). The presence of myocardial 18F-fluorodeoxyglucose (18F-FDG) uptake has been promoted to be one of the major criterions. With complete suppression of physiologic myocardial accumulation by proper patient preparation, 18F-FDG uptake represents the presence of active sarcoid lesions in the myocardium (3, 4). However, CS patients with heart failure (HF) frequently show diffuse 18F-FDG uptake in the myocardium even under sufficient preparation. HF has been reported to increase myocardial dependence on glucose metabolism and alter energy metabolism from free fatty acids (FFAs) to glucose (5). This alteration would cause diffuse 18F-FDG uptake in the myocardium and makes it difficult to differentiate pathologic 18F-FDG uptake from physiologic myocardial 18F-FDG accumulation in the failing heart. The evaluation of perfusion-metabolic mismatch comparative to myocardial perfusion single photon emission computed tomography (SPECT) is a supportive way to confirm latent pathologic 18F-FDG uptake under diffuse physiologic accumulation in the myocardium (6). The aim of this study was to verify the clinical value of myocardial perfusion SPECT images for detecting active inflammatory lesions in CS with diffuse myocardial 18F-FDG uptake.
We studied 39 consecutive patients with CS who underwent 18F-FDG PET/CT and resting myocardial perfusion SPECT at our institution. All patients underwent endomyocardial biopsy (EMB). Ten patients had histologically proven CS. The remaining 29 patients were clinically diagnosed with CS based on the JCS guidelines (2). Coronary angiography was also performed in all patients to exclude coronary artery disease. The Ethical Committee for the Clinical Research of National Cerebral and Cardiovascular Center approved this study.
18F-FDG PET imagingAll patients followed a high-fat and low-carbohydrate diet (7) with prolonged fasting (8) prior to their 18F-FDG PET/CT scan. After establishing for venous access, serum glucose was evaluated and 18F-FDG (2 to 2.5 MBq/kg body weight) was injected. 18F-FDG PET/CT imaging was performed on a 40-slice PET/CT system (Biograph mCT, Siemens Medical Solutions USA Inc. PA, USA) with three-dimensional acquisition at 60 minutes after 18F-FDG administration. Transmission scanning for low-dose CT as part of PET/CT was performed for attenuation correction.
Patterns of 18F-FDG uptake were divided into three groups: “none,” “focal,” and “diffuse.” “None” was defined as no 18F-FDG uptake in the myocardium. Patients with focal areas of increased 18F-FDG uptake with complete suppression of physiologic 18F-FDG accumulation were classified as having “focal” uptake (Figure 1A and B). Diffuse homogeneous or heterogeneous myocardial 18F-FDG uptake was defined as “diffuse” uptake (Figure 1C and D). Focal-on-diffuse 18F-FDG uptake pattern was included in “diffuse” uptake because physiologic myocardial accumulation showed several patterns (9). There was no clear standard to distinguish focal-on-diffuse from diffuse 18F-FDG uptake.
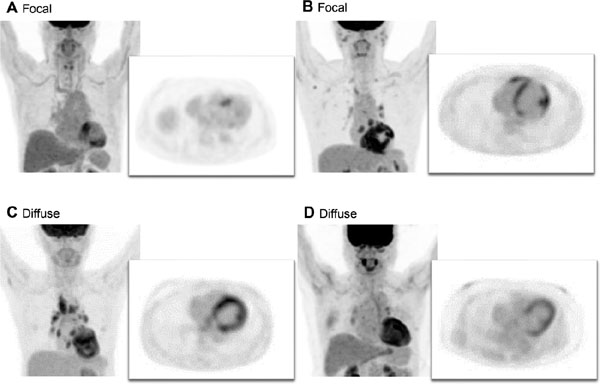
Focal and diffuse 18F-FDG uptake in the myocardium.
A, and B are three-dimensional MIP and transverse plane images from cardiac 18F-FDG PET in patients with focal 18F-FDG uptake. C and D are three-dimensional MIP and transverse plane images of diffuse 18F-FDG accumulation in the myocardium.
18F-FDG, 18F-fluorodeoxyglucose; MIP, maximum intensity projection; PET, positron emission tomography
Cardiac 18F-FDG PET polar maps were generated using Quantitative Perfusion SPECT software (QPS; Cedars-Sinai Medical Center, Los Angeles, CA, USA).
Myocardial perfusion SPECT imagingElectrocardiogram-gated resting myocardial perfusion SPECT with 99mtechnetium (99mTc) -labeled tracer was performed with a dedicated solid-state camera containing cadmium zinc telluride semiconductor detectors (D-SPECT; Spectrum Dynamics Medical, Caesarea, Israel) within a month of the 18F-FDG PET scan. Depending on body weight, patients received 296–370 MBq of 99mTc-labeled tracer intravenously. Myocardial perfusion SPECT polar maps were created using QPS for simultaneous evaluation with cardiac 18F-FDG PET polar maps. The severity of perfusion abnormalities was visually interpreted using the five-point scale for scoring as follows; 0, normal perfusion; 1, mild reduction in counts; 2, moderate reduction in counts; 3, severe reduction in counts; and 4, absent radiotracer uptake (10). Perfusion abnormalities with a score of 3 to 4 were defined as “perfusion defect.” When the myocardial perfusion defect corresponded to the focal areas of increased 18F-FDG uptake, it was defined as perfusion-metabolic mismatch. Two experienced nuclear radiologists interpreted the presence of perfusion-metabolic mismatch and when their evaluations differed, an agreement was reached by discussion.
Left ventricular (LV) function was automatically calculated based on gated SPECT using Quantitative Gated SPECT software (QGS; Cedar-Sinai Medical Center, Los Angeles, CA, USA).
Statistical analysisAll continuous variables are presented as means±SD. Unpaired t-tests were used to compare groups. Analysis of variance was used to compare means across multiple groups. Categorical variables are presented as percentages and were compared using the χ2 test. All statistical tests were two-sided and p values <0.05 were regarded as statistically significant. Statistical analysis was performed with JMP version 13.0.0 (SAS Institute, Cary, NC, USA).
Increased myocardial 18F-FDG accumulation was observed in 33 (84.6%) patients while 6 (15.4%) patients did not have any myocardial 18F-FDG uptake. Two patients with negative 18F-FDG uptake in the myocardium had been treated before 18F-FDG PET scan was performed. Other 4 patients were probably in the stage of scarring after active inflammation spontaneously settled down. There was no significant difference in the duration of fasting between patients with increased myocardial 18F-FDG accumulation and those without. Extracardiac sarcoid involvement was found in 18 patients by 18F-FDG PET. Out of these 18 patients, extracardiac biopsy was performed in one patient with cutaneous sarcoidosis.
Of 33 patients with increased myocardial 18F-FDG accumulation, focal 18F-FDG uptake was detected in 16 patients and diffuse 18F-FDG uptake was observed in 17 patients. Table 1 shows the characteristics of patients in the focal and diffuse groups. LV ejection fraction was lower in the diffuse group than in the focal group (p=0.015). Serum brain natriuretic peptide (BNP) levels within a week before and after the 18F-FDG PET/CT scan were significantly higher in the diffuse group than in the focal group (p=0.002). More patients in the diffuse group showed myocardial perfusion defect compared with the focal group (p=0.021). Only one patient in the diffuse group treated with both prednisolone and methotrexate because of the repetition in recurrence. There was no significant difference in the percentage of having received corticosteroid therapy between the focal and diffuse group.
The median duration between 18F-FDG PET and 99mTc-SPECT was 1 day (IQR −16 to +26 days). All patients with perfusion defect in the focal group demonstrated perfusionmetabolic mismatch compared with 50% in the diffuse group (Figure 2). Figure 3A showed a typical perfusion-metabolic mismatch pattern in a patient with focal 18F-FDG uptake. A myocardial perfusion defect corresponded to focal area of increased 18F-FDG uptake from the anterior wall to the septum of the left ventricle. Figure 3B and 3C were two representative 18F-FDG uptake patterns in the diffuse group. In the case depicted in Figure 3B, myocardial perfusion defects were observed in the same locations as increased 18F-FDG uptake. This perfusion–metabolic mismatch seemed to indicate active inflammatory CS lesions. In Figure 3C, active inflammation in the lateral wall of the left ventricle could not be excluded based on the heterogeneously increased myocardial 18F-FDG uptake. However, no active inflammation was demonstrated on the lateral wall of the left ventricle because no perfusion–metabolic mismatch was detected. Papillary muscle uptakes may have played a role to stand out the lateral 18F-FDG uptake. Although diffuse 18F-FDG uptake was generally classified as non-specific pattern and defined as negative for active CS lesion, the evaluation of perfusion-metabolic mismatch in diffuse myocardial 18F-FDG accumulation could estimate the latent active inflammatory lesions in CS.

Prevalence of perfusion–metabolic mismatch in the focal and diffuse groups.

Representative examples of perfusion-metabolic mismatch and physiologic myocardial 18F-FDG accumulation (perfusion-metabolic match).
A: Sixties female clinically diagnosed CS. BNP level was slightly increased (26.3pg/ml) and LV function was mildly depressed (LVEF=40%). Focal 18F-FDG uptake from the anterior wall to the septum of the left ventricle corresponded to a myocardial perfusion defect (arrows).
B: Fifties female histologically diagnosed isolated CS. LV function was severely depressed (LVEF=22%) and BNP level was 700pg/ml. Although diffuse myocardial 18F-FDG accumulation was observed, it occurred in locations with myocardial perfusion defects. This was interpreted as perfusion-metabolic mismatch (arrows). A year after starting corticosteroid therapy, LVEF improved from 22% to 37% and no myocardial 18F-FDG uptake was detected in the follow-up 18F-FDG PET scan. Active inflammation of CS was latent in physiologic myocardial 18F-FDG accumulation.
C: Fifties male clinically diagnosed isolated CS. BNP level was 111.7 pg/ml and LV function was severely depressed (LVEF=20%). Focally increased 18F-FDG uptake under diffuse myocardial accumulation was observed in the inferolateral wall; whereas, it did not correspond to regions with myocardial perfusion defects. Diffuse 18F-FDG uptake might represent residual viable myocardium, not active inflammation of CS.
BNP: brain natriuretic peptide, CS: cardiac sarcoidosis, 18F-FDG: 18F-fluorodeoxyglucose, LV: left ventricular, LVEF: left ventricular ejection fraction; MIP, maximum intensity projection; PET, positron emission tomography; QPS, Quantitative Perfusion SPECT software, 99mTc-MIBI: 99mtechnetium-sestamibi.
Diffuse physiological 18F-FDG uptake in the myocardium is frequently occurred due to inadequate preparation. However, under the high-fat and low-carbohydrate diet with about 18 hours fasting prior to their 18F-FDG PET/CT scan, diffuse myocardial 18F-FDG uptake was observed in CS patients with lower LV ejection fraction and higher BNP levels. With simultaneous evaluation of myocardial 18F-FDG PET and 99mTc- SPECT polar maps, half of CS patient with diffuse myocardial 18F-FDG uptake showed perfusion-metabolic mismatch as active inflammatory lesions of CS. The assessment of perfusion-metabolic mismatch would be necessitated for evaluating disease activity in CS patients with diffuse myocardial 18F-FDG uptake, who had advanced HF.
18F-FDG PET is a promising modality for detecting increased glucose metabolism, which is a hallmark of immune cell activation in CS. According to a meta-analysis published in 2012, 18F-FDG PET has a sensitivity of 89% and a specificity of 78% for the diagnosis of CS (11). With complete suppression of physiologic myocardial accumulation, focal 18F-FDG uptake in the myocardium indicates the upregulation of glucose metabolism at sites of macrophage-mediated inflammation (12). However, there are several caveats to the evaluation of CS disease activity by using 18F-FDG PET alone because myocardium utilizes a mixture of glucose and FFAs for energy metabolic substrates (13). Even with proper patient preparation, 22% to 38% of individuals still have the physiologic myocardial 18F-FDG accumulation (14). In addition, physiologic myocardial 18F-FDG accumulation is uncontrollable due to the alteration of myocardial FFAs and glucose metabolism in HF (15, 16). When the ventricle is overloaded, the fetal gene program would be activated in order to maximize efficiency and decrease oxygen consumption. Thus, FFAs utilization decreases while glycolysis increases in the failing heart. In this study, we have found that patients with diffuse 18F-FDG uptake had more advanced HF, as evidenced by lower LV function and higher BNP levels than patients with focal 18F-FDG uptake. When diffuse 18F-FDG uptake is observed in patients with CS who have HF, the assessment of perfusion-metabolic mismatch is indispensable.
Perfusion–metabolic mismatch in the myocardium is a sign of active inflammation in CS because the presence of inflammation leads to compression of the microvasculature and scar-related fibrosis (17). In the present study, 79% of patients with myocardial 18F-FDG uptake showed myocardial perfusion defects and 55% presented perfusion-metabolic mismatch. Additionally, 100% of patients with focal 18F-FDG uptake and myocardial perfusion defect demonstrated perfusion-metabolic mismatch. Although focal 18F-FDG uptake in the lateral wall and the base of the LV has been frequently observed in healthy humans (18), the assessment of perfusion-metabolic mismatch would be valuable to distinguish abnormal 18F-FDG uptake in inflammatory myocardial sarcoid lesions from physiologic myocardial accumulation. Furthermore, it should be noted that perfusion-metabolic mismatch was also detected in half of patients with diffuse 18F-FDG uptake in the myocardium. Without the comparison of 18F-FDG PET and myocardial perfusion SPECT, the single evaluation of 18F-FDG PET may lead to a false-negative conclusion for CS disease activity in patients with HF.
The expert consensus groups of the Society of Nuclear Medicine and Molecular Imaging and the American Society of Nuclear Cardiology (ASNC) have recommended assessing two sets of images, 18F-FDG and myocardial perfusion images, to identify active inflammatory lesions in CS (19). Myocardial perfusion PET imaging with 82Rubidium or 13N-ammonia is preferred since they minimize radiation exposure. However, 82Rubidium is not approved by Japan's Pharmaceutical Affairs and 13N-Ammonia is only covered for ischemic heart disease by Japanese national health insurance. Resting myocardial 99mTc-SPECT imaging is more widely available than myocardial PET imaging. Although perfusion defects on myocardial perfusion scintigraphy is included in the minor criteria in JCS guidelines for diagnosis of CS (2), only 18 patients underwent myocardial 99mTc-SPECT before 18F-FDG PET scan in this study. In most cases, coronary angiography was performed to exclude ischemic heart disease. Due to advances in SPECT scanner technology, myocardial perfusion 99mTc-SPECT imaging can be obtained with an effective radiation dose of approximately 3 mSv. While SPECT has lower spatial resolution than PET, we generated polar maps of both PET and SPECT images using the same software and algorithm. The comparison of these polar maps made the identification of perfusion-metabolic mismatch simpler and easier than comparing PET with SPECT slices side-by-side using a conventional cardiac display.
Diffuse 18F-FDG uptake tends to be observed in patients with advanced HF under proper preparation. It sometimes presents latent active inflammatory lesions under physiologic myocardial accumulation and at other times demonstrates residual viable myocardium. Since it remains challenging to achieve complete suppression of myocardial 18F-FDG accumulation in HF, simultaneous evaluation of 18F-FDG PET and 99mTc-SPECT imaging by polar map analysis could help to detect latent active inflammation in CS. Identification of perfusion-metabolic mismatch provides more clinically relevant information and could avoid misestimating CS disease activity in HF.
Study limitationsSeveral limitations of this study should be mentioned. First, this study was performed at a single institution and included a relatively small number of patients. Second, patients with both treated and untreated CS were included. Steroid therapy, the gold-standard treatment for CS, may alter blood glucose levels and affect physiologic myocardial 18F-FDG accumulation. Third, this study was a retrospective observational study and there was potential for sampling bias. Plasma FFAs and insulin levels were not measured in all patients. The time lag between 18F-FDG PET and 99mTc-SPECT scan was inappropriate. Since the medical condition of CS has changed from moment to moment, the same day evaluation of 18F-FDG PET and 99mTc-SPECT scan is desired to improve the diagnostic accuracy. Furthermore, there was a possibility for inadequate avoidance of carbohydrate-containing foods in our study population and it might be the cause of diffuse 18F-FDG uptake. The ASNC imaging guidelines recommend the abundance of carbohydrate-containing foods should begin about 24 hours prior to the test, with an intake of high-fat and high-protein foods for at least two meals 24 hours prior (19). Our study population avoided carbohydrate-containing foods about 18 hours prior to the test and took high-fat and high-protein foods only one meal and then an overnight fast. Since all 18F-FDG PET scans were performed on outpatients with the national health insurance, it was not simple and easy to encourage patients for overnight fasting and the avoidance of carbohydrate-containing for about 24 hours prior to the test. The simultaneous evaluation of 18F-FDG and 99mTc-SPECT can be also helpful to distinguish active inflammation from physiological myocardial 18F-FDG uptake caused by inadequate preparation. Finally, we did not assess patient outcomes or examine the relationship between perfusion–metabolic mismatch and clinical prognosis. A further prospective study in a larger population is warranted in order to establish the beneficial effect and prognostic value of examining perfusion–metabolic mismatch in patients with CS.
ConclusionDiffuse myocardial 18F-FDG accumulation is often seen in patients with HF and it may cause underestimation of CS disease activity or misidentification as active inflammation associated with CS. Characterizing disease activity based on 18F-FDG uptake alone requires great caution. This study highlights the usefulness of myocardial perfusion 99mTc-SPECT images for assessing myocardial 18F-FDG uptake in patients with CS who have HF. The assessment of perfusion–metabolic mismatch is valuable for the optimal evaluation of CS disease activity in the presence of diffuse myocardial 18F-FDG uptake.
The authors thank all nuclear medicine staff members for their assistance in the preparation of the manuscript.
None.
None of the authors have any conflicts of interest to declare.