2022 Volume 8 Issue 1 Pages 14-20
2022 Volume 8 Issue 1 Pages 14-20
Abstract
Purpose: Heart-type fatty acid binding protein (H-FABP) is primary transporter of free fatty acid and plays an important role in myocardial metabolism, which is characterized by high specificity and rapid appearance under ischemic condition. The objective of this study was to clarify the usefulness of imaging study of targeting H-FABP appearance using radio-labeled antibody, and correlation with myocardial fatty acid metabolism and perfusion in acute reperfusion ischemia.
Method: Wistar rats were allotted to sham-operated control group (sham; n=4), ischemia non-reperfused group (IG; n=5), and ischemia-reperfusion group (RG; n=5). Ligation of left coronary artery (LCA) was performed for IG and RG. 20 min of ischemia was followed by 60min of reperfusion for RG. 125I labeled anti H-FABP antibody (anti H-FABP), BMIPP and 99mTc-sestamibi (MIBI) was injected intravenously. Multi-tracer digital autoradiogram was performed using µ-imager®. The ratio of radioactivity in LCA related (culprit) area to the inferior (remote) area (target uptake ratio=TUR) was generated.
Results: In sham group, no visually detectable accumulation was observed for the anti H-FABP image, and TURMIBI and TURBMIPP were equivalent to 1. In IG, TURMIBI and TURBMIPP were remarkably low (0.12±0.01, 0.24±0.07). In RG, TURMIBI was significantly lower (0.20±0.03, p<0.05 vs. other groups). However, TURBMIPP was significantly higher (2.78±1.28, p<0.05) compared to the sham and IG, whereas anti H-FABP showed markedly higher ratio in the reperfused area compared to the sham and IG (3.43±0.73 vs. 0.31±0.13 and 1.09±0.07 for IG and sham; p<0.05, and <0.01, respectively).
Conclusion: Anti H-FABP accumulated specifically in reperfused area under acute ischemia, and it accorded to the area where fatty acid metabolism was activated. This study has shown the future potential for clinical application in vivo imaging of acute coronary syndrome.
The early triage and therapeutic decision making for patients suspected acute coronary syndrome is common challenging and has been expected to improve. Cardiac biomarker such as creatinine kinase type MB (CK-MB), myoglobin, troponin T (TnT), or I (TnI), which are specifically localized in myocardium is now an accurate and useful diagnostic tool for acute myocardial infarction (AMI) than conventional process such as anamnesis, physical examination, electrocardiogram, or echocardiography. In emergency medicine, measuring cardiac biomarker is quite useful and recommended for detecting unstable angina (UA) or non-ST elevated AMI (1). But these markers are still lacking in diagnostic performance in detecting initial several hours from onset (2).
Recently, heart-type fatty acid binding protein (H-FABP) has been focused as most accurate diagnostic marker for acute coronary syndrome, which is specifically localized in myocardium and thought to leak out in quite early phase of acute ischemia (3). Detecting methodology of H-FABP by using immuno-histochemical assay has been established and employed to clinical use (4–6). The detection of acute coronary syndrome using H-FABP has been introduced and its diagnostic ability was confirmed. Commercially available detection kits for H-FABP is now frequently used in outpatient clinics and the emergency departments (7–9). But several clinical trials reported that this method still has issue in lacking of specificity and low positive predictive value compared to conventional biomarkers (10–14).
As well as biomarker test, noninvasive cardiac imaging in emergency cardiology has been employed frequently with the help of innovating technology. In some facilities, the imaging study by using myocardial fatty acid analogue are set up for detecting the patients with acute coronary syndrome and this approach is quite useful combined and compared with radiopharmaceutical perfusion agent (15, 16). For detecting myocardial fatty acid metabolism dysfunction would be a key to find high-risk patient, cardiac fatty acid imaging is discordant in acute phase of ischemia. Therefore, we hypothesized that imaging study targeting FABP would be a precursor for detection of early phase of ischemia.
In this study, we aimed to explore the altering in myocardial fatty acid transporting system in acute reperfusion ischemia by using imaging of fatty acid transporter and evaluate its potency for clinical application.
Materials and methods
Preparation of radiopharmaceuticals
MIBI and BMIPP were supplied by Fuji RI pharmaceuticals Co., Ltd., (Tokyo, Japan) and Nihon-Medi-physics (Kobe, Japan) as lyophilized kit formulations. 125I was supplied by Perkin-elmer (Waltham, MA) as I-Na in vial kit. Anti-heart type fatty acid binding protein antibody (Anti-H-FABP antibody [diluted 1:2,500] Abcam Co., Ltd., Cambridge, UK). The conventional iode-gen method was employed for radiolabeling. The methodology was previously described by (17). The purity of radiolabeled antibody was evaluated by thin layer chromatography, and was over 90% at 2 hours post labeling under 37 degree.
Bio-distribution study
Healthy rats (n=4) were employed to bio-distribution study. 40 min after tracer injection, heart, lung, brain, liver, kidney and quadriceps were removed. Radioactivity of each organ and 1cc of whole blood were counted by well counter (ALOKA). Measured data were presented as counts per min (cpm/g). Decay correction for measured activity was not performed due to long half-life.
Rat acute ischemia reperfusion model
Male wistar rats (aged 6 to 8 weeks, body weight 250–300 g n=13; Saitama Experimental Animals Supply Co., Ltd. Saitama, Japan) were anesthetized by intraperitoneal injection of pentobarbital (25 mg/kg), and kept under mechanical ventilation with room air. After left thoracotomy, anterior wall of the rat heart was in the view. Myocardial ischemia was induced by ligation in left coronary artery by a 5-0 polypropylene suture. Myocardial ischemia was confirmed by change of the appearance in anterior wall and motion. Sham-operated group (Sham n=4) was enrolled as control group. The schematic of protocols was shown in Figure 1. Coronary ligation was performed for 20 min, then ligation was released and followed by 40 min of reperfusion (ischemia-reperfusion group=RG, n=5). Permanent occlusion was performed for ischemia non-reperfusion group (IG n=5). During reperfusion or occlusion (approximately 40 min after reperfusion, or 60 min after ligation or sham operation), the tracers were administrated via tail vain (5MBq of FABP for protocol 1, n=4; 50, 15, and 5MBp of MIBI, BMIPP, and FABP for protocol 2, n=5 for ischemia after reperfusion). The experimental protocol was in accordance with the institutional guidelines.

Image acquisition by multi-tracer digital autoradiogram
Micro-imager® (Biospace lab co., ltd., Lyon, France) was employed to perform digital autoradiogram. In protocol 1, 20 min after tracer injection, rats were sacrificed, and the heart was quickly removed. The heart was embedded in optimal cutting temperature compound (Tissue-tek®, Sakura-fintec, Japan) and frozen by liquid-nitrogen. Short axis slices (20 µm) of mid ventricle was obtained on a microtome (Cryostat CM3050®, Leica, Microsystems co., ltd., Mannheim, Germany). 3×3 cm of Scintillator sheet® (Biospace) was covered on section. 10 hours of acquisition was performed by Micro-imager®.
For multi-tracer digital autoradiogram, a preliminary study was performed for 99mTc, 123I, and 125I to evaluate the accuracy of isotope separation. 1×1×1 cm of 4 frozen cubes, which contained 10, 20, 30 µCi/mL of each radio-isotope, and mixed all tracers were made. Frozen cubes were cut into 20 µm of slices by using a microtome and covered by scintillator sheet for image acquisition. A commercially available software (Multi Decay Label: MDL®, Biospace lab co., ltd., Lyon, France), which enables to separate isotope concentration by half-life of radio-isotope was employed to obtain the image of individual tracer. A preset setting was employed according to the experiment by Riou et al. (18). As shown in Figure 2, 40 hours of acquisition is enough to obtain satisfactory separated images. Thus, 40 hours of acquisition was performed after protocol has finished. All data was analyzed by using M3 vision (Biospace lab), and myocardial uptake (cpm/mm2) and target uptake ratio (TUR) was obtained for the ratio of myocardial uptake in reperfused area against remote myocardium (Figure 3A).
To confirm the specificity of the imaging using H-FABP antibody, co-injection with excess amount of mouse-IgG (1:500, Abcam) labeled by 125I was performed for sham (n=1) and RG (n=1) by same procedure. 10 hours of digital autoradiogram was performed, and analyzed using same setting as described above.


Statistical analysis
All values are expressed as mean±SD, and are compared with nonparametric data. A Windows software tool, GraphPad PRISM® (version 5.0, Graph Pad, NC) was used to analyze the generated data. A one-factor ANOVA and post-hoc test were used in an attempt to compare the generated data. A value of p<0.05 was considered statistically significant.
Results
Bio-distribution of radiolabeled anti H-FABP antibody
Radioactive counts in organs were shown in Table 1. Radioactive in blood was quite low. The heart count was specifically higher compared to lung. Relatively higher activity was observed in liver and kidney. A remarkably low uptake was observed in brain.

Image of ant H-FABP in acute ischemia-reperfusion
The tracer accumulation was observed in ischemia-reperfused area (TURFABP=2.1±0.05, n=4, Figure 3B left upper). In contrast, homogeneous accumulation was observed in the sham group. The specificity study using 125I labeled non-specific mouse IgG showed no accumulation in the ischemia-reperfused area (Figure 3 right upper).
Comparison to myocardial perfusion and fatty acid metabolism by multi-tracer autoradiogram
In sham operated group, the target uptake ratio (TUR) of all tracers was equivalent to 1 (0.1±0.10, 1.0±0.08, and 1.09±0.07 for TURMIBI, TURBMIPP, and TURFABP; n=4). In IG, TURMIBI, TURBMIPP, and TURFABP were remarkably low compared to sham (0.12±0.01, 0.24±0.07, and 0.31±0.13). In contrast, significant higher uptake was observed in ischemia-reperfused area (TURFABP=3.47±1.50) in RG (Figure 4). In comparison among three groups, statistically significant increased TUR was observed in RG for TURBMIPP and TURFABP compared to other groups in the area of LCA related territory where TURMIBI significantly reduced (Figure 4 right).
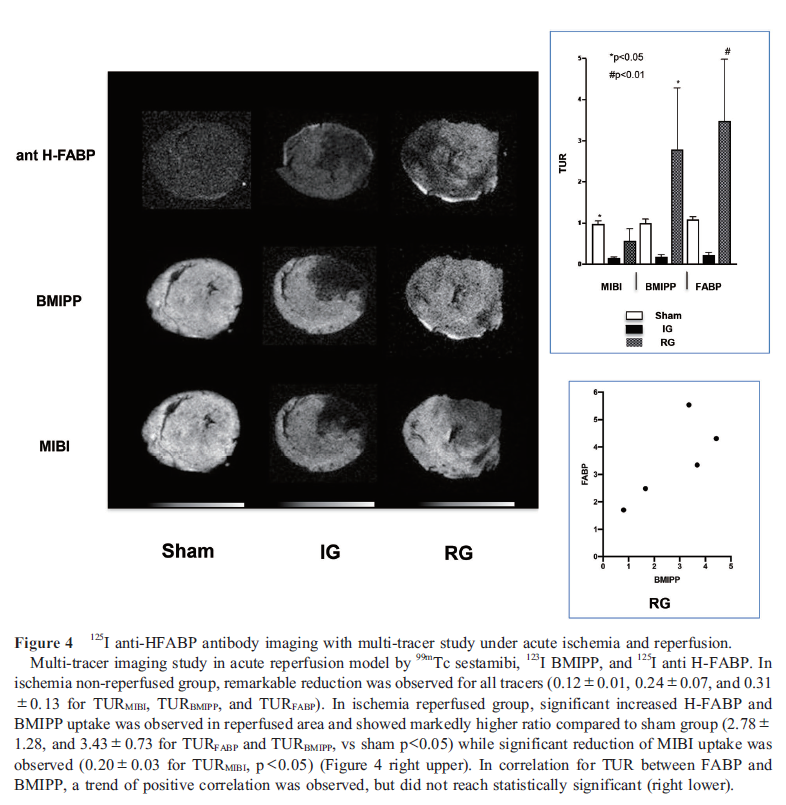
Discussion
In this study, we evaluated the potency of imaging in myocardial fatty acid transporting system in acute reperfusion ischemia using radiolabeled anti H-FABP antibody. The images showed significant accumulation in reperfused-ischemic myocardium, which indicated the future potential for the non-invasive tool as “hot-spot imaging”. FABP is thought to specifically express in myocardium and consists of two subtypes. One is expressed on myocardial membrane in a bound form with FAT/CD36+ and engaged in incorporating free fatty acid into myocardial cells. The other is mainly retained in cytosol, which is combined with incorporated free fatty acid and transported into triglyceride pools and mitochondria (19, 20). These proteins are different in their molecular weight (FABP on membrane is 40 kDa, the other is 15 kDa), and the anti-body we used in this study is targeting cytosolic FABP (21). It is widely known that FABP is easy to leak out from myocardium and pooled in extra cellular spaces in the early phase of ischemia. Considering molecular weight, anti H-FABP is monoclonal antibody, which is usually incapable of passing through myocardial membrane; impossible to visualize intra-myocardial H-FABP in normal myocardium. Fatty acid binding protein family was known for various subgroups, which specifically expressed in liver, intestine, testis, brain, and muscle including myocardium (14). Our result in bio-distribution study showed specific accumulation in heart compared to other organs due to specific subtypes of the protein. A subtype of FABP is also expressed in brain, but blood brain barrier would protect antibody from reaching targeting protein. This is the reason why tracer distribution in brain is low. High activity in liver was also observed, but this is unknown whether antibody reacted liver type FABP or purely under metabolite. A substantial uptake was also observed in quadriceps, it might reflect the existence of FABP in muscle.
Using FABP in early detection of acute coronary syndrome in emergency department allows more accurate risk stratification compared to the guidance of conventional diagnosis. Cardiodetect® is frequently used in bedside in emergency medicine in clinical setting, which enable us to detect high risk patients and multi-center study is under investigation (22). However, Haltern et al. reported that blood test of FABP is lacking its specificity for non-ST elevated AMI (11). Seino et al. reported that blood testing with H-FABP demonstrated high sensitivity and low specificity in initial hours after onset of acute coronary syndrome (5). There are several issues in specificity due to the existence of various types of FABP and H-FABP also express in other organs such as major vessel. And also, the accuracy of FABP testing frequently influenced by the age or underlying disease such as renal dysfunction, post surgery state, and skeletal muscle injury (23, 24). In emergency medicine, it is frequent to encounter the patients with those status. With this respect, the imaging study using high-sensitive marker can be powerful probe for triage. For this imaging study was performed using digital autoradiography, the detectability of this imaging technique should be validated in preclinical setting using small animal SPECT system to determine whether it can detect small size of infarction.
In multi-tracer digital autoradiogram, there were significant differences in myocardial uptake for all three tracers. Myocardial perfusion, and especially myocardial mitochondrial function is thought to be easily insulted by ischemia or hypoxia. In our study, the uptake of MIBI was significantly decreased in area of occluded or reperfused myocardium, indicating myocardial mitochondrial dysfunction. Nevertheless, combined with fatty acid metabolism study, significant accelerated BMIPP uptake was observed. This phenomenon seemed to be controversial, however, several reports proved that fatty acid metabolism is enhanced early phase of ischemia-reperfusion. In clinically, several discordant findings of BMIPP and flow tracers were reported (25). Seino et al. reported that fatty acid uptake tracer showed greater accumulation compared perfusion tracer in some of the patients with myocardial infarction and unstable angina who received reperfusion therapy (5). In experimental setting using rat, Noriyasu et al. reported BMIPP uptake was relatively higher compared to thallium-201 in ischemic myocardium (26). Higuchi et al. reported BMIPP uptake was higher compared to myocardial MIBI uptake for 72 hours in reperfused myocardium then started to decrease after 3 days (27). Using isolated rat heart model, we previously reported that BMIPP extraction was accelerated immediate after 15 min of acute ischemia and more acceleration was observed in 30 min, while extraction of perfusion tracer decreased (28). In large animal study, Nishimura et al. documented that BMIPP uptake was higher than TL perfusion in reperfused myocardium, but similar reduction was observed in permanent occlusion group (29). They concluded that the reason of enhancement of BMIPP uptake was due to the nature of the tracer, which is designed to resist beta oxidation would predominantly retain triglyceride pools. BMIPP may reflex over stored fatty acid in TG pool or intra-cellular and not assess true myocardial beta oxidation in myocardial mitochondria (26, 27). Further studies are needed to explore the acceleration and correlation of BMIPP and anti H-FABP in acute reperfused ischemia.
In translating this novel technique into clinical application, several issues should be addressed. First, a stability and metabolic fate of radiolabeled HFABP is unknown. In general, monoclonal antibody is known to be metabolized in the liver, renal clearance, or both. The eliminating half-life of HFABP is reported to be approximately 270 min, thus the imaging feasibility should be limited in several hours after onset (3). Our biodistribution study revealed remarkable hepatic uptake which indicated a considerable liver metabolism. Biodistribution in large mammals including human also needs to be investigated. Second, the safety of monoclonal antibody administration needs to be discussed. The clinical usefulness and safety of monoclonal antibody imaging has been investigated since 1980. These studies have been investigated mainly oncologic fields, mostly, In-111 or 123I have been used as primal radionuclides. We used 125I labeled mouse monoclonal antibody which may have significant risks for antibody-mediated side-effects including an anaphylaxis, a late allergic reaction or production of neutralizing antibodies. The draggability for human origin antibody needs to be tested in the process of translating into clinical practice. Currently, the safety of antibody treatment has been extensively investigated because of the recent emergence of theranostics, and the technological innovation in introducing humanized antibody with less immunogenicity has been widely established (30). It can be expected that this promising technique will be introduced into non-invasive imaging. Nevertheless, although the further studies are needed to clarify the usefulness of this in-vivo antibody imaging, the results of our study indicated a potential to proceed to highly specific hot-spot imaging modality in emergency medicine.
Conclusion
Imaging study using radiolabeled anti-FABP antibody is feasible to identify acutely ischemic myocardium, and indicated the future potential for clinical application in-vivo imaging.
Acknowledgment
We thank HONYAKU-town for english editing.
Sources of funding
None.
Conflicts of interest
None.
References
1. Anderson PA, Malouf NN, Oakeley AE, Pagani ED, Allen PD. Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ Res 1991; 69: 1226–33.
2. Balk EM, Ioannidis JP, Salem D, Chew PW, Lau J. Accuracy of biomarkers to diagnose acute cardiac ischemia in the emergency department: a meta-analysis. Ann Emerg Med 2001; 37: 478–94.
3. Tanaka T, Hirota Y, Sohmiya K, Nishimura S, Kawamura K. Serum and urinary human heart fatty acid-binding protein in acute myocardial infarction. Clin Biochem 1991; 24: 195–201.
4. Nakata T, Hashimoto A, Hase M, Tsuchihashi K, Shimamoto K. Human heart-type fatty acid-binding protein as an early diagnostic and prognostic marker in acute coronary syndrome. Cardiology 2003; 99: 96–104.
5. Seino Y, Ogata K, Takano T, et al. Use of a whole blood rapid panel test for heart-type fatty acid-binding protein in patients with acute chest pain: comparison with rapid troponin T and myoglobin tests. Am J Med. 2003; 115: 185–90.
6. Alansari SE, Croal BL. Diagnostic value of heart fatty acid binding protein and myoglobin in patients admitted with chest pain. Ann Clin Biochem 2004; 41: 391–6.
7. Ishii J, Wang JH, Naruse H, et al. Serum concentrations of myoglobin vs human heart-type cytoplasmic fatty acid-binding protein in early detection of acute myocardial infarction. Clin Chem 1997; 43: 1372–8.
8. Alhashemi JA. Diagnostic accuracy of a bedside qualitative immunochromatographic test for acute myocardial infarction. Am J Emerg Med 2006; 24: 149–55.
9. Seino Y, Tomita Y, Takano T, Ohbayashi K. Office cardiologists cooperative study on whole blood rapid panel tests in patients with suspicious acute myocardial infarction: comparison between heart-type fatty acid-binding protein and troponin T tests. Circ J 2004; 68: 144–8.
10. Charpentier S, Ducassé JL, Cournot M, et al. Clinical assessment of ischemia-modified albumin and heart fatty acid-binding protein in the early diagnosis of non-ST-elevation acute coronary syndrome in the emergency department. Acad Emerg Med 2010; 17: 27–35.
11. Haltern G, Peiniger S, Bufe A, Reiss G, Gülker H, Scheffold T. Comparison of usefulness of heart-type fatty acid binding protein versus cardiac troponin T for diagnosis of acute myocardial infarction. Am J Cardiol 2010; 105: 1–9.
12. Hjortshøj S, Kristensen SR, Ravkilde J. Diagnostic value of ischemia-modified albumin in patients with suspected acute coronary syndrome. Am J Emerg Med 2010; 28: 170–6.
13. Valle HA, Riesgo LG, Bel MS, Gonzalo FE, Sanchez MS, Oliva LI. Clinical assessment of heart-type fatty acid binding protein in early diagnosis of acute coronary syndrome. Eur J Emerg Med 2008; 15: 140–4.
14. Storch J, McDermott L. Structural and functional analysis of fatty acid-binding proteins. J Lipid Res 2009; 50 Suppl: S126–31.
15. Kawai Y, Tsukamoto E, Nozaki Y, Morita K, Sakurai M, Tamaki N. Significance of reduced uptake of iodinated fatty acid analogue for the evaluation of patients with acute chest pain. J Am Coll Cardiol 2001; 38: 1888–94.
16. Kontos MC, Dilsizian V, Weiland F, et al. Iodofiltic acid I 123 (BMIPP) fatty acid imaging improves initial diagnosis in emergency department patients with suspected acute coronary syndromes: a multicenter trial. J Am Coll Cardiol 2010; 56: 290–9.
17. Michael Conlon J. Preparation of 125I-labeled peptides and proteins with high specific activity using IODO-GEN. In: Walker JM, editor. The protein protocols handbook. 2nd edition, Totowa, NJ: Humana Press; 2002. p. 971–7.
18. Riou LM, Broisat A, Lartizien C, et al. Assessment of non-reperfused and reperfused myocardial infarction using diffusible or deposited radiolabelled perfusion imaging agents. Eur J Nucl Med Mol Imaging 2007; 34: 330–7.
19. Hirai T, Nohara R, Ogoh S, et al. Serial evaluation of fatty acid metabolism in rats with myocardial infarction by pinhole SPECT. J Nucl Cardiol 2001; 8: 472–81.
20. Bonen A, Luiken JJFP, Glatz JFC. Regulation of fatty acid transport and membrane transporters in health and disease. Mol Cell Biochem 2002; 239: 181–92.
21. Heather LC, Cole MA, Lygate CA, et al. Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovasc Res 2006; 72: 430–7.
22. Bruins Slot MH, van der Heijden GJ, Rutten FH, et al. Heart-type Fatty acid-binding protein in Acute Myocardial infarction Evaluation (FAME): background and design of a diagnostic study in primary care. BMC Cardiovasc Disord 2008; 8: 8.
23. Górski J, Hermens WT, Borawski J, Mysliwiec M, Glatz JFC. Increased fatty acid-binding protein concentration in plasma of patients with chronic renal failure. Clin Chem 1997; 43: 193–5.
24. Van Nieuwenhoven FA, Kleine AH, Wodzig WH, et al. Discrimination between myocardial and skeletal muscle injury by assessment of the plasma ratio of myoglobin over fatty acid-binding protein. Circulation 1995; 92: 2848–54.
25. Tamaki N, Kawamoto M, Yonekura Y, et al. Regional metabolic abnormality in relation to perfusion and wall motion in patients with myocardial infarction: assessment with emission tomography using an iodinated branched fatty acid analog. J Nucl Med. 1992; 33: 659–67.
26. Noriyasu K, Mabuchi M, Kuge Y, et al. Serial changes in BMIPP uptake in relation to thallium uptake in the rat myocardium after ischaemia. Eur J Nucl Med Mol Imaging 2003; 30: 1644–50.
27. Higuchi T, Taki J, Nakajima K, Kinuya S, Namura M, Tonami N. Time course of discordant BMIPP and thallium uptake after ischemia and reperfusion in a rat model. J Nucl Med 2005; 46: 172–5.
28. Fukushima K, Momose M, Kondo C, Hagiwara N, Sakai S. Accelerated BMIPP uptake immediately after reperfused ischemia in the isolated rat heart model. Ann Nucl Med 2011; 25: 560–5.
29. Nishimura T, Sago M, Kihara K, et al. Fatty acid myocardial imaging using 123I-beta-methyl-iodophenyl pentadecanoic acid (BMIPP): comparison of myocardial perfusion and fatty acid utilization in canine myocardial infarction (occlusion and reperfusion model). Eur J Nucl Med 1989; 15: 341–5.
30. Dammes N, Peer D. Monoclonal antibody-based molecular imaging strategies and theranostic opportunities. Theranostics 2020; 10: 938–55.