Article ID: 24-00001
Article ID: 24-00001
Abstract
Background: This study investigated the feasibility of using a quantitative diagnostic method based on 99mTc-pyrophosphate scintigraphy (PYP) lateral planar images to differentiate between PYP-positive (myocardial uptake) and false-positive (blood pool uptake) scans.
Methods: The study included 93 consecutive patients with suspected transthyretin amyloid cardiomyopathy (ATTR-CM) who underwent PYP between April 2022 and December 2023. Patients were categorized using planar anterior PYP images according to the Perugini visual grading system; patients with grades ≥2 were analyzed. Whether the uptake of the ventricle was in the blood pool or the myocardium was confirmed by transaxial single-photon emission tomography (SPECT). The heart-to-mediastinum ratios (H/M ratio) of the left lateral planar images at 1- and 3-h were calculated by placing a circular region of interest in the heart and cephalodorsal side of the heart to determine optimal cut-off values.
Results: Among the PYP images, the study analyzed 15 positives diagnosed as ATTR-CM and 10 false positives. Significant differences were observed in the H/M ratio at 1- and 3-h (both p < 0.01), with 100% sensitivity and specificity using cut-off values of 1.22 and 1.21 at 1- and 3-h, respectively. The interclass correlation coefficients (2, 1) between the two readers were 0.919 and 0.958 for the 1- and 3-h H/M ratios, respectively.
Conclusions: Lateral planar PYP imaging can exclude PYP false-positive cases caused by blood pools in a simple and quantitative manner using only a 1-h planar image, possibly obviating the need for SPECT imaging.
Cardiac amyloidosis (CA) is a progressive and severe disease characterized by the deposition of amyloid fibers in the myocardium and is associated with poor prognoses, including heart failure and fatal arrhythmias (1, 2). Transthyretin amyloid cardiomyopathy (ATTR-CM), the most frequent form of CA, is caused by cardiac deposition of insoluble amyloid fibrils formed by misfolded transthyretin proteins (3). CA was once considered a rare condition; however, because of widespread disease awareness, the development of diagnostic techniques, and an aging population, it is increasingly being diagnosed, especially in patients with aortic stenosis, heart failure with a reduced ejection fraction, and hypertrophic cardiomyopathy (3, 4).
For the diagnosis of ATTR-CM, 99mTc-Pyrophosphate scintigraphy (PYP) is recommended in several diagnostic guidelines and expert consensus documents (5–7). Myocardial uptakes of grade 2 (equal to rib uptake) or grade 3 (greater than rib uptake) in PYP or the heart-to-contralateral (H/CL) ratio (cardiac accumulation counts in the denominator and contralateral lung field accumulation in the numerator) of >1.5 at 1-h, or >1.3 at 3-h have high sensitivity and specificity (3, 8–10). As a result, a definitive diagnosis of ATTR-CM, previously requiring endomyocardial biopsy, can now be made noninvasively using PYP, allowing ATTR-CM diagnosis in facilities and patients where endomyocardial biopsy is difficult to perform (8).
False-positive results have been reported for diagnostic methods using PYP planar images alone because of a blood pool in the heart or an overlying bone artifact. Therefore, single-photon emission tomography (SPECT) or SPECT/CT imaging is recommended to confirm myocardial uptake and avoid false positives (5, 11–13); however, not all hospitals use SPECT/CT or SPECT. In addition, qualitative assessment by SPECT alone might make it difficult to determine whether accumulation occurs in the myocardium or blood pool.
One solution to this problem may be to use the blood pool instead of the lungs as the H/CL denominator. However, because of strong accumulation in the sternum, placing regions of interest (ROIs) in blood pools such as the aorta and pulmonary artery is difficult in the PYP anterior view. Therefore, a lateral planar image may exclude the sternum and place the ROI in the large blood vessels. To address this issue, this study investigated the feasibility of quantitative ATTR-CM assessment from lateral planar PYP images of suspected ATTR-CM using SPECT as a reference standard.
Materials and methods
Patients
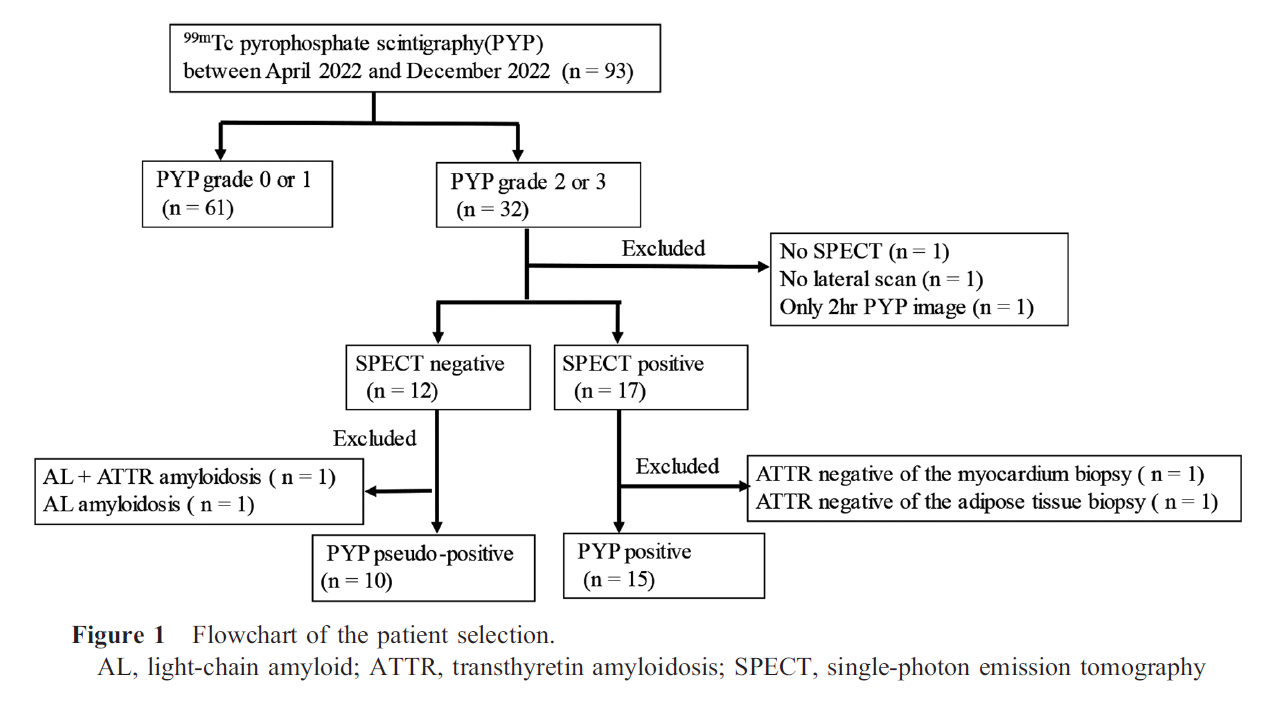
This single-center retrospective study enrolled 93 consecutive patients with suspect or known transthyretin amyloidosis (ATTR) who underwent PYP between April 2022 and December 2023. The patient enrollment is described in Figure 1. Patient demographics and echocardiography findings were collected from medical records. This study was approved by the Institutional Review Board of Fukushima Medical University (No. REC2023-030).
PYP-planar and SPECT
PYP-planar imaging and SPECT were performed using a 3-head gamma detector SPECT system (GCA 9300R; Canon Medical Systems, Tokyo, Japan) equipped with low-energy high-resolution collimators. For the PYP imaging protocol, 370 MBq of PYP was administrated intravenously. The patients were on standby for approximately 60 and 180 min for acquisition. Anterior, left anterior oblique, and left lateral planar acquisitions were performed for 4 min, followed by SPECT. The energy window was 140 KeV ±20%. Scatter corrections were not applied. The parameters for SPECT acquisition were a 64×64 matrix, four angles for step-and-shoot with 90 views in a circular orbit, and a zoom of 1.0; the back projection was filtered with a 0.41 Butterworth filter for reconstruction (14).
Image analysis
PYP planar images were assessed for visual grading in 93 consecutive patients, using Perugini scores to classify PYP scans (10). Among the patients with a Perugini score of 2 or 3, whether the uptake of the ventricle was a blood pool or myocardium was confirmed by transaxial SPECT imaging. Images with uptake in the blood pool were defined as false positives; uptake in the left ventricular myocardium was defined as positive PYP images. The H/CL ratio of the PYP anterior planar images and the heart-to-mediastinum (H/M) ratio of the lateral planar images at 1- and 3-h were calculated. The H/CL ratio was defined as the ratio of the heart ROI mean count to the contralateral lung count on the PYP anterior planar image. The H/M ratio was defined as the ratio of the heart ROI to the mean mediastinal ROI count on the PYP left lateral planar image. A circular ROI of the anterior planar image was drawn in the left ventricle, according to Bokhari et al. (9, 15). In the left lateral image, a circular ROI was placed with the largest size not exceeding the area presumed to be accumulation in the left ventricle. In the mediastinum, a circular ROI was placed on the superior dorsal side of the left ventricle at a location thought to be the ascending aorta so as not to exceed the aortic accumulation. The images were assessed by two experimental radiologists.
Statistical analysis
The normality of each group’s data was evaluated using the Shapiro–Wilk test. Parametric variables are represented as the mean ± standard deviation, and non-parametric variables are represented as the median with range; comparisons were performed using Student’s t-test or the Mann–Whitney U test, respectively. Categorical variables are expressed as numbers and percentages; comparisons used the chi-squared test.
The interclass correlation coefficients (ICCs; 2 and 1) were calculated to evaluate the H/M ratio. Receiver operating characteristic (ROC) analysis was performed to discriminate between the H/M ratios of the ATTR-positive and false-positive groups. Optimal cut-off values were determined based on the maximal sum of sensitivity and specificity (Youden index). The Spearman correlation coefficient was plotted for the H/CL and H/M ratios in the ATTR-positive and false-positive groups. A value of p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (version 28.0; SPSS, Chicago, IL, USA).
Results
Among the 93 patients, 32 were grade 2 or 3; 10 false-positive and 15 positive PYP images were included in the analysis. The analysis excluded PYP images without a lateral scan (n=1), without a SPECT image (n=1), images at 2 h (n=1), SPECT-negative with a biopsy result of AL (light-chain amyloid) amyloidosis (n=1), SPECT-negative with a biopsy result of ATTR+AL (n=1), SPECT-positive but ATTR-negative by myocardial biopsy (n=1), and SPECT-positive but ATTR-negative by an adipose tissue biopsy (n=1) (Figure 1). ATTR-CM was diagnosed using an endomyocardial biopsy (wild type: 9; no genetic testing: 3) and non-invasive methods (n=3) among the 15 patients with ATTR-CM.
Table 1 presents the demographics and statistical results of the patients in the positive and false-positive groups. Significant differences between the two groups were observed in H/CL ratios at 1 and 3 h (both p < 0.01), H/M ratios at 1- and 3-h (both p < 0.01), Perugini grade (p < 0.01), interventricular septum thickness (p < 0.01), and posterior LV wall thickness (p < 0.01).

The areas under the ROC curve were 1.0 for the H /M ratio at 1- and 3-h. The cut-off value of the Youden index was 1.22 for 1-h and 1.21 for 3-h; both had 100% sensitivity and specificity (Figure 2). The ICCs (2, 1) between the two readers were 0.919 and 0.958 for the 1- and 3-h H/M ratios, respectively. Pearson’s correlation analysis revealed a weak correlation between the H/CL and H/M ratios at 1-h (r = 0.41) and 3-h (r = 0.40) in the PYP-positive group; a moderate correlation was observed at 1-h (r = -0.51) in the PYP false-positive group; no correlation was observed in the PYP false-positive group at 3-h (r = -0.03). Figures 3 and 4 present representative cases comparing the H/CL and H/M ratios in the positive and false-positive cases.



Discussion
This study investigated the diagnostic value of 1- and 3-h lateral planar PYP images in patients with suspected ATTR-CM using SPECT as a reference standard. The H/M ratio in PYP left lateral images was lower than the H/CL ratio in PYP anterior images. This result may be explained by the fact that the denominator of the H/CL ratio in the anterior image is the lung parenchyma, whereas the denominator of the H/M ratio in the lateral image is the blood pool, which has a greater count than the lung. The H/CL ratio threshold consensus for 3-h is lower than that for 1-h. This result may indicate a lower blood pool at 3-h compared to 1-h. In contrast, the threshold of the H/M ratio for 1- and 3-h was comparable (1.22 and 1.21, respectively). This finding may indicate that the H/M ratio is less affected by the blood pool.
Positive and false-positive cases were quantitatively distinguishable in both the 1- and 3-h PYP lateral planar images because the denominator uses a blood pool, such as the aorta or pulmonary artery. Therefore, if the cause of cardiac accumulation is the blood pool, the H/M ratio should be close to 1. In contrast, if PYP images show accumulation in the myocardium, the H/M ratio should be high even when divided by the blood pool.
The challenge with this method is that the location of the ROI is difficult in cases where cardiac vs. mediastinum PYP accumulation is unclear. The H/M ratio in patients with Perugini grade 0 and 1 is likely to be variable depending on the number of ribs included in the ROI because of difficulties in visualizing the heart and aorta and the small amount of accumulation other than bone. Therefore, this analysis method should not be used for all cases. Calculating the H/M ratio by adding a lateral image is suggested in equivocal cases where accumulation is evident but not conclusively positive. An algorithm can be considered in which cases with grade 0 or 1 on PYP anterior images are excluded as negative, and cases with grade 2 or greater are evaluated by PYP lateral planar images instead of SPECT or SPECT/CT. This study did not include false-positive patients who had a grade-3 Perugini score. Therefore, evaluation with PYP lateral images of only Perugini grade 2 cases may be sufficient to avoid false-positive results. This method can rule out false positives with a short PYP lateral image collection time, even in facilities where SPECT is difficult to perform. Furthermore, the 1- and 3-h H/M ratios distinguished equally and completely between positive and false positives in the current study; therefore, PYP imaging at 3-h can be omitted. The H/M ratio may also be useful in avoiding false negatives of the H/CL ratio; for example, in cases where the H/CL ratio showed a low value due to high accumulation in the contralateral lung field (such as the overlapping of vertebrae in a patient with scoliosis) in the true positive case.
Uptake by the residual blood pool, skeletal muscle, and overlying bone may cause PYP false positives; however, renal dysfunction and accumulation in skeletal muscle have not been reported to be important factors in overestimating PYP imaging (5, 16–18). In addition to ATTR-CM, other diseases present with PYP accumulation, including myocarditis, myocardial ischemia, hyperphosphatemia, and hydroxychloroquine cardiomyopathy (19–22). These diseases might cause false positives; however, they cannot be excluded from PYP lateral image analysis because they involve PYP accumulation in the myocardium, and the blood pool is not the cause of false positives.
Limitations
The first limitation of this study is its single-institutional, retrospective nature and the low number of included patients. The false-positive rate was relatively high compared with previous studies, possibly because of the bias of single-institution studies, including inter-reader differences and the many cases of aortic stenosis before transcatheter aortic valve implantation included in this study. Therefore, a multicenter study with a larger sample size is warranted. Second, the positioning of the ROI sites for measuring the heart and mediastinum was challenging, and differences in the H/M ratio were observed, especially in cases where no visual uptake was observed in the cardia. Moreover, ROI positioning may cause variations in the location and size of the ROI placed on the mediastinum in slender patients in which the gap between the vertebra and the sternum may not be as wide. Third, patients with false-positive PYP are often older adults for whom biopsies are not readily available; therefore, excluding patients with mild cardiac amyloidosis was difficult since ATTR-CM was excluded based on PYP-SPECT, ultrasound, and clinical findings.
Conclusion
This study shows that lateral planar PYP images can be used to exclude PYP false-positive cases caused by blood pools in a simple, quantitative manner and may obviate the need for SPECT imaging. Furthermore, 1-h PYP images showed good sensitivity and specificity similar to 3-h images; therefore, 3-h images could be omitted.
Acknowledgments
None.
Sources of funding
None.
Conflicts of interest
None.
References
1. Garcia-Pavia P, Rapezzi C, Adler Y, et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2021; 42: 1554–68.
2. Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: A review. J Am Heart Assoc 2012; 1: e000364.
3. Tahara N, Lairez O, Endo J, et al. 99mTechnetium-pyrophosphate scintigraphy: A practical guide for early diagnosis of transthyretin amyloid cardiomyopathy. ESC Heart Fail 2022; 9: 251–62.
4. Treibel TA, Fontana M, Gilbertson JA, et al. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: Prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging 2016; 9: e005066.
5. Dorbala S, Ando Y, Bokhari S, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2—evidence base and standardized methods of imaging. Circ Cardiovasc Imaging 2021; 14: e000029.
6. Kittleson MM, Ruberg FL, Ambardekar AV, et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2023; 81: 1076–126.
7. Kitaoka H, Izumi C, Izumiya Y, et al. JCS 2020 guideline on diagnosis and treatment of cardiac amyloidosis. Circ J 2020; 84: 1610–71.
8. Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133: 2404–12.
9. Castano A, Haq M, Narotsky DL, et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: Predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol 2016; 1: 880–9.
10. Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 2005; 46: 1076–84.
11. Asif T, Gomez J, Singh V, Doukky R, Nedeltcheva A, Malhotra S. Comparison of planar with tomographic pyrophosphate scintigraphy for transthyretin cardiac amyloidosis: Perils and pitfalls. J Nucl Cardiol 2021; 28: 104–11.
12. Dorbala S, Ando Y, Bokhari S, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 2 of 2—diagnostic criteria and appropriate utilization. Circ Cardiovasc Imaging 2021; 14: e000030.
13. Poterucha TJ, Elias P, Bokhari S, et al. Diagnosing transthyretin cardiac amyloidosis by technetium Tc 99m pyrophosphate: A test in evolution. JACC Cardiovasc Imaging 2021; 14: 1221–31.
14. Suenaga H, Fukushima K, Ishii S, et al. Global and regional reduction of myocardial perfusion in patients with transthyretin type of cardiac amyloidosis. Ann Nucl Cardiol 2023; published online. doi: 10.17996/anc.23-00009
15. Bokhari S, Castaño A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. 99mTc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 2013; 6: 195–201.
16. Hutt DF, Quigley AM, Page J, et al. Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur Heart J Cardiovasc Imaging 2014; 15: 1289–98.
17. Saitou T, Aikawa T, Manabe O, Nagase A, Kudo T, Oyama-Manabe N. Comparison of 1-h with 3-h planar 99mTc-pyrophosphate scintigraphy in patients with suspected transthyretin cardiac amyloidosis using SPECT as a reference standard. Ann Nucl Med 2023; 37: 99–107.
18. Sperry BW, Gonzalez MH, Brunken R, Cerqueira MD, Hanna M, Jaber WA. Non-cardiac uptake of technetium-99m pyrophosphate in transthyretin cardiac amyloidosis. J Nucl Cardiol 2019; 26: 1630–7.
19. Hu LH, Kuo Y, Chang FP, et al. Hyperphosphatemia-related false-positive 99mTc-pyrophosphate myocardial scan: A case report with endomyocardial biopsy result. Clin Nucl Med 2023; 48: e544–6.
20. Wells RG, Ruskin JA, Sty JR. Myocardial imaging. Coxsackie myocarditis. Clin Nucl Med 1986; 11: 661–2.
21. Khalaf S, Khan N, Al-Mallah M, Kassi M, Shah D. A positive PYP scan: Thinking beyond amyloid. J Nucl Cardiol 2021; 28: 1796–7.
22. El-Tallawi KC, Parikh R, Nabi F, Maclayton PI, Trachtenberg BH, Al-Mallah M. A positive Tc-99m PYP scan in a patient with cardiac sarcoidosis. J Nucl Cardiol 2021; 28: 2390–94.