Abstract
Purpose: This study evaluated the discoloration of current flowable and universal resin composites by immersing in black tea over 30 days.
Materials and Methods: Three flowable resin composites and three universal resin composites were evaluated. The composites were inserted into a disk-shaped stainless steel mold and properly cured. The surfaces of the composite disks were wet-ground and ultrasonically cleaned. Colors – L*, a*, and b* were measured at the center of disks on a gray background using a spectrophotometer. The disks were immersed in black tea at 37˚C for 30 days, and the colors were repeatedly measured at 1, 3, 5, and 30 days of immersion. Color differences ΔE*ab were calculated from the L*, a*, and b* values. Water sorption and solubility of the composites were also measured as per ISO 4049. The results were statistically analyzed, and regression analyses were done between ΔE*ab and ΔL*, Δa*, Δb* or sorption/solubility.
Results: All the composites showed observable increases of ΔE*ab within the first 5 days of immersion. Values of ΔE*ab ranged from 0.64 to 4.97, and three composites exhibited values above the clinically acceptable value, 3.3. ΔL* and Δb* revealed strong correlations with ΔE*ab. Both water sorption and solubility had positive correlations with ΔE*ab at 30 days.
Conclusion: Tea immersion induced discoloration of the current resin composites. This discoloration was affected most by the change in brightness and difference in its blue-yellow chromaticity, and the level of discoloration was material dependent.
Introduction
Adhesive restoration is a conventional technique for the reconstruction of tooth structure lost to dental caries/fracture. Among the adhesive restorative materials, resin composites have wide ranges of clinical applications including direct restorations, core build-ups, and luting of indirect restoratives. Light-cured resin composites, in particular, are essential for direct restorations for of their adhesiveness, mechanical and esthetic properties [1]. Resin composites first began to be supplied in the form of pastes (hereinafter referred to as universal resin composite). Subsequent compositional alterations have produced less viscous resin composites, known as flowable resin composites since 1996 [2]. Flowable composites are dispensed by a syringe through a needle tip, achieving easy handling for filling relatively small cavities or cavities with large undercuts [3,4].
Initially, flowable composites contained hybrid-type fillers that were considerably larger than the current fillers [2]. The filler content was low with approximately 25 wt% to attain sufficient flowability of the composites, making their mechanical properties inferior to those of universal resin composites. Hence, flowable composites were mainly used for small cavities or as cavity liners [2,5]. Subsequent developments in filler technology, i.e., surface treatments and the dispersion techniques produced much smaller fillers [6], which improved the mechanical properties of composites. In addition, viscosity of composites became controllable due to filler/monomer technology. The flowable composites became applicable in occlusal load bearing areas and allowed for contouring the anatomical forms under the direct syringe application. With such improvements, clinical usage of flowable composites have increased in present day [7].
As described earlier, low viscosity is an advantage of the flowable composites. Flowability is controlled by the compounding base resins. The major monomer is bisphenol A-glycidyl methacrylate (Bis-GMA) for current composites due to its mechanical strengths. However, as Bis-GMA is too viscous to use alone in composite in terms of manipulation, other monomers such as urethane dimethacrylate (UDMA) and triethylene glycol dimethacrylate (TEGDMA), and certain surfactants are compounded to adjust the viscosity [6,8]. Different base resins are frequently utilized in flowable and universal composites of the same manufacturer. Several studies have investigated discoloration of resin composites [7,9,10,11], and the degree of discoloration has reportedly depended on the compositions of resin composites [10,11,12,13].
The objective of this study was to compare the discoloration of current flowable and universal composites by extrinsic pigments — black tea. In addition, water sorption and solubility of the composites, which were significant factors affecting the discoloration [14], were measured and their relations to the discoloration were analyzed. The null hypothesis tested was that levels of discoloration differ between flowable and universal composites.
Materials and Methods
Materials
Table 1 lists six resin composites evaluated in this study and their basic formulations — three flowable resin composites: Clearfil Majesty ES Flow (MA, Kuraray Noritake Dental, Tokyo, Japan), GC MI Fil (MI, GC, Tokyo, Japan), and Filtek Supreme Ultra Flow (SU, 3M ESPE, St. Paul, MN, USA), and three universal resin composites: Clearfil Majesty ES-2 (ma, Kuraray Noritake Dental), GC Gracefil (mi, GC), and Filtek Supreme Ultra (su, 3M ESPE). A3 shade was selected for all composites. Comparing the filler contents between flowable and universal composite of the same manufacturer, MA and ma exhibited almost the same amount while MI and SU contained approximately 13 wt% more fillers than mi and su, respectively. Regarding the main base resins, three manufacturers used different resins for their flowable and universal composites as mentioned in the introduction.
Table 1 Resin composites evaluated in this study
| Tradename |
Manufacturer |
Code |
Filler (wt%) |
Component |
Lot No. |
| Flowable type |
| Clearfil Majesty ES Flow |
Kuraray Noritake Dental |
MA |
75 |
silanated barium glass, silanated silica, TEGDMA, hydrophobic aromatic dimethacrylate |
CQ0075 |
| GC MI Fil |
GC |
MI |
69 |
strontium glass, UDMA |
1504011 |
| Filtek Supreme Ultra Flow |
3M ESPE |
SU |
65 |
ytterbium trifluoride, silica, zirconia/silica cluster, Bis-GMA, TEGDMA, other methacrylates |
N658998 |
| Universal type |
| Clearfil Majesty ES-2 |
Kuraray Noritake Dental |
ma |
78 |
silanated barium glass, pre-polymerized organic filler, Bis-GMA, other methacrylic acid type monomer |
BE00554 |
| GC MI Gracefil |
GC |
mi |
82 |
barium glass, Bis-MEPP, UDMA |
1502182 |
| Filtek Supreme Ultra |
3M ESPE |
su |
78.5 |
silica, zirconia, zirconia/silica cluster, Bis-EMA, Bis-GMA, UDMA |
N655239 |
TEGDMA, triethylene glycol dimethacrylate; UDMA, urethane dimethacrylate; Bis-GMA, bisphenol-A-diglycidyl methacrylate; Bis-MEPP, bisphenol-A-ethoxylate dimethacrylate; Bis-EMA, ethoxylated bisphenol-A-glycol dimethacrylate
Discoloration
Each resin composite was inserted into a stainless steel mold (7 mm in diameter, 3 mm in thickness) on a glass plate. The composites were covered with a transparent plastic strip (9.5 mm in width, 3M ESPE) and a glass plate. The composites were irradiated from the top surface using a quartz-tungsten-halogen (QTH) light (Optilux 501 sds Kerr, Orange, CA, USA) at 650 mW/cm2 for 20 s with the light-guide tip contacting with the glass plate. The top and bottom surfaces of the composite disks were wet-ground with #1200-grit SiC paper under finger pressure for 15 s. Following ultrasonic cleaning for 1 min, colors (CIE Lab system) of the disks were measured at the center of the disks using a spectrophotometer (SE-2000, Nippon Denshoku Industries, Tokyo, Japan) on a gray background.
Black tea was used for the discoloration test. The tea solutions (Ceylon tea, Coop, Tokyo, Japan) were prepared using three tea bags (2 g each) and 250 mL of boiling water for 5 min. After leaving the tea for 15 min at room temperature, 1 mL of tea was poured into 3.4-mL wells. The composite disks were immersed in the tea at 37˚C for 30 days. All the disks were kept in separate wells and the tea was changed every week. At 1, 3, 5, and 30 days of immersion, the disks were ultrasonically cleaned for 15 s and their color was measured following the same method as described above. Differences (ΔL*, Δa*, and Δb*) were obtained from the respective values before and after immersion, and the color differences ΔE*ab were calculated using the following equation (1).
-
ΔE*ab = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2 (1)
The resulting data of ΔE*ab were statistically analyzed using Mann-Whitney U test at α = 0.05 (n = 5). The statistics were analyzed using IBM SPSS Statistics ver.23 (IBM, Tokyo, Japan). Simple regression analyses were performed between ΔE*ab and ΔL*, Δa*, or Δb*.
Water sorption and water solubility
Water sorption and water solubility of the resin composites were measured as per ISO 4049. The composites were inserted into a stainless steel mold (15 mm in diameter, 1 mm in thickness) between glass plates and plastic strips. Irradiation was completed using the same curing device from the top and bottom surfaces for 160 s. Flat surfaces of the disks were wet-ground with #600-grit SiC paper to adjust the thickness to 1 ± 0.01 mm, and the volume of the disks (V) were calculated. The specimens were kept in a desiccator at 37˚C. After 22-hour storage, the specimens were stored in a second desiccator at 23˚C for 2 hours and then weighed (HR-251AZ, A&D, Tokyo, Japan). These procedures were repeated until a constant weight (m1) was obtained. The disks were then immersed in a distilled water at 37˚C for 1 week. Excess water was removed by blotting and the disks were weighed (m2). The specimens were re-stored in the 37˚C desiccator and repeatedly weighed until the constant weight (m3) as described. The following equations (2 and 3) were used for calculating the water sorption (Wsp) and water solubility (Wsl).
-
Wsp = (m2 – m3) / V (2)
-
Wsl = (m1 – m3) / V (3)
The resulting data, Wsp and Wsl were statistically analyzed using Mann-Whitney U test at α = 0.05 (n = 5). Simple regression analyses were also done between ΔE*ab at 30 days and Wsp or Wsl.
Results
Discoloration
ΔE*ab
Figure 1 lists the changes of color difference (ΔE*ab) during the immersion period. All the composites exhibited the increases of ΔE*ab over the immersion period. ΔE*ab ranged from 0.64 (MA 1-day) to 4.97 (su 30-day). MA showed the lowest means at every interval of measurement. ΔE*ab above the acceptable value (>3.3, [15]) were yielded at 30-day of SU, mi and su. The same composites (SU, mi and su) demonstrated significant increases of ΔE*ab from 1-day to 30-day. Statistical comparisons were also performed between the values of 1-day and 5-day, and of 5-day and 30-day as all the composites were observed to show inflection in their line graphs at 5 days. Within the 5-day immersion, ΔE*ab significantly increased in the composites except SU and mi. After the 5-day immersion, 2 composites (SU and su) revealed significant increases of the values.

Fig. 1 Changes in ΔE*ab over 30-day immersion: A, Flowable resin composite; B, Universal resin composite
Figure 2 illustrates the changes in differences in brightness (ΔL*) during the immersion. All the composites demonstrated negative values of ΔL* at each measurement, appeared darker, and showed obvious decreases within the 5-day immersion. The values ranged from −0.41 (MA 1-day) to −3.29 (su 30-day). Figure 3 exhibits the scatter diagram and the result of regression analysis. ΔE*ab and ΔL* exhibited a negative correlation with 0.8602 of the coefficient (r).
Δa* (chromaticity: green - red)
Figure 4 reveals the changes of differences in chromaticity (Δa*) during the immersion. The values ranged from −0.12 (MA 1-day) to 0.71 (MI 5-day and 30-day). All the composites were observed to exhibit that the changes within the first 5 days were less obvious than those in ΔL*. Figure 5 demonstrates the result of regression analysis. ΔE*ab and Δa* exhibited a weak positive correlation with r = 0.3114.
Δb* (chromaticity: blue - yellow)
Figure 6 illustrates the changes of differences in brightness (Δb*) during the immersion. The values ranged from −0.36 (MA 3-day) to 3.61 (su 30-day). MA, mi and su revealed apparent increases of Δb* after 5 days of immersion, which behavior was similar to those in ΔE*ab. Figure 7 exhibits the result of regression analysis. ΔE*ab and Δb* exhibited a positive correlation with r = 0.8293.

Fig. 2 Changes in ΔL* over 30-day immersion: A, Flowable resin composite; B, Universal resin composite

Fig. 3 Result of regression analysis between ΔE*ab and ΔL*
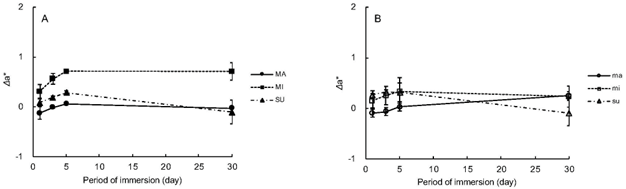
Fig. 4 Changes in Δa* over 30-day immersion: A, Flowable resin composite; B, Universal resin composite
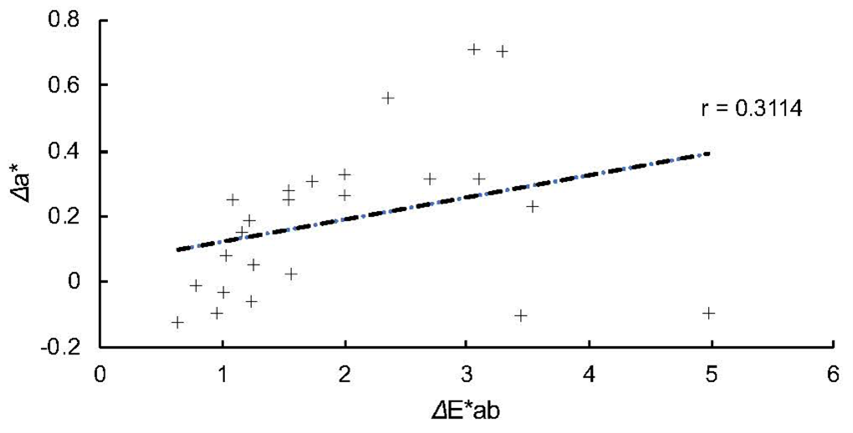
Fig. 5 Result of regression analysis between ΔE*ab and Δa*
Table 2 lists water sorption (Wsp μg/mm3) and water solubility (Wsl μg/mm3) of the six resin composites. Means of Wsp ranged from 15.69 (MA) to 28.38 (su). The value of MA was significantly the smallest while su exhibited the greatest value among the composites. Means of Wsl ranged from 0.79 (MA) to 2.62 (su). MA, MI and mi demonstrated significantly smaller values than SU and su. Figures 8 and 9 illustrates the results of regression analyses. Both Wsp and Wsl exhibited weak positive correlations with ΔE*ab at the same level of the coefficients (0.5962 and 0.5764), respectively.

Fig. 6 Changes in Δb* over 30-day immersion: A, Flowable resin composite; B, Universal resin composite
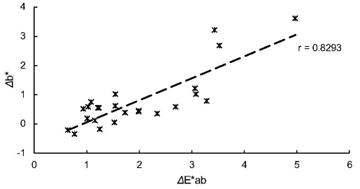
Fig. 7 Result of regression analysis between ΔE*ab and Δb*
Table 2 Water sorption (
Wsp) and water solubility (
Wsl) of the resin composites
| Code |
Type |
Wsp (μg/mm3) |
Wsl (μg/mm3) |
| MA |
Flowable |
15.69 (1.25)A |
0.79 (1.37)a |
| MI |
Flowable |
22.17 (0.62)B |
1.18 (0.45)a |
| SU |
Flowable |
22.41 (0.50)B |
2.18 (0.46)b |
| ma |
Universal |
22.90 (1.34)B |
1.86 (0.75)ab |
| mi |
Universal |
17.38 (0.59)C |
1.14 (0.23)a |
| su |
Universal |
28.38 (0.77)D |
2.62 (0.66)b |
Values having the same superscript are not statistically different in the same column (n = 5; Mann-Whitney U-test, p > 0.05).

Fig. 8 Result of regression analysis between ΔE*ab at 30 days and Wsp
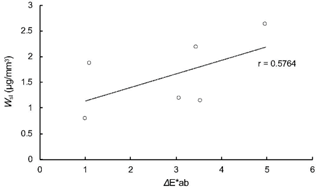
Fig. 9 Result of regression analysis between ΔE*ab at 30 days and Wsl
Discussion
Adhesive restorations are essential for achieving minimally invasive dentistry, and resin composites play a major role in direct restorations [16]. As mentioned in the introduction, flowable resin composites have evolved with significant improvements in its physical properties – to levels similar to that of universal composites, and as a result, together with its ease in handling, have increased its clinical application [4,7]. Advances in filler/monomer technologies have also helped the color stability of composites, however have not improved to degrees in which discoloration is prevented altogether [9,10,11], and remain a reason for repair [17]. In this study, discolorations of six composites (three flowable, three universal) were examined after immersion in black tea.
ΔE*ab is a numerical indication of the color difference obtained by calculating using brightness (ΔL*) and chromaticity (Δa* and Δb*). Previous study indicated color difference to be visible to the human eye when ΔE*ab was greater than 3.3 [15]. Among the composites used in this study, SU from the flowable composite group, and mi and su from the universal composite group showed a difference greater than 3.3 at 30 days of immersion. Figure 1 shows increase in ΔE*ab in all composites up to 5 days of immersion, but the values only continued to increase up to 30 days of immersion in three composites; SU, mi, and su. This suggested that discoloration from black tea progressed in all composites up to 5 days of immersion, regardless of their differences in composition. However, in comparing the composition of composites in which ΔE*ab increased even after 5 days (SU, mi, and su), and that of composites that showed no significant increase after 5 days (MA, MI, and ma), no common factor could be identified within the groups. It is suggested that discoloration therefore is affected by various factors such as filler particle size and type of base resin used being complexly intertwined. Level of the discoloration was material dependent and could not be supposed with the type of composite, flowable or universal. Thus, the hypothesis was rejected.
Water sorption and water solubility have been reported to be factors influencing discoloration in composites [14]. With Wsp and Wsl measured in this study, MA exhibited the smallest mean values among the composites – supporting the ΔE*ab results, where MA showed the smallest values at all timings of the color measurement. Considering the relations between water sorption and filler content, the sorption reportedly decreased as the filler content increased in mono-filler composites [18]. The universal composites (ma, mi, and su) reveal the same or higher level of filler content than MA, however, they demonstrated statistically greater Wsp than MA. In addition, the current composites contain several kinds of fillers as shown in Table 1. This suggests that prediction of water sorption by its filler/base resin contents to be difficult. With composites having similar filler content like MA and ma, while MA contains TEGDMA, a hydrophilic base resin as its main component, ma contains Bis-GMA – a hydrophobic base. Water sorption of Bis-GMA was reported to be greater than that of TEGDMA [19], agreeing with this study’s results in ΔE*ab and Wsp. This suggests the type of base resin affects the discoloration in composites [7]. In addition, single linear regression analysis of ΔE*ab-Wsp and ΔE*ab-Wsl, both showed a positive correlation of r = 0.6, supporting that water sorption and water solubility were true factors affecting discoloration in composites. This can also be assumed from the ΔE*ab value of MA at 30 days after immersion in black tea, as it showed the lowest value among the 6 composites studied and had the lowest Wsp and Wsl.
ΔL* indicate brightness, showing color change in dark - light. At 1 day after immersion, all composites showed negative ΔL* values, indicating that colors turned darker. This change in color resulted in a similar pattern as ΔE*ab, and mostly occurred up to 5 days of immersion. Δa* and Δb* shows color difference in saturation; from green – red and blue – yellow direction, respectively. Similar to ΔL*, changes in Δa* occurred mostly within 5 days of immersion, while Δb* showed similar behavior with ΔE*ab. The three factors, ΔL* and Δb* showed a strong correlation with ΔE*ab.
As black tea contains yellow-red polyphenol pigment, it can be speculated that this pigment stained the surface of the composite specimen right after immersion, and discoloration progressed with the water sorption of the base resin. Considering the sorption speed of the base resin, initial discoloration most likely was due to the polyphenol pigments staining the surface of the composites. In Karadas’ study, when flowable composites were immersed in black tea for 7 days and ΔL*, Δa*, and Δb* were measured, Δb* showed a larger value than Δa* [7]. As per Um and Ruyter’s study, for composites immersed in black tea set at 50˚C for 4 months, the Δb* increased, and speculated that yellow pigments to be more easily absorbed by resins, due to its polarity compatibility [20]. While our study was performed under different conditions from these previous studies, through the 30-day immersion, Δb* showed increase while ΔL* decreased – agreeing with the previous theories stated. It has become more apparent that with immersion in black tea, composites appear yellowish and darker.
Conflicts of Interest
None
References
- 1. Heintze SD, Rousson V, Mahn E. Bond strength tests of dental adhesive systems and their correlation with clinical results –A meta-analysis. Dent Mater 2015; 31: 423-34.
- 2. Bayne SC, Thompson JY, Swift EJ Jr, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. J Am Dent Assoc 1998; 129: 567-77.
- 3. Estafan D, Schulman A, Calamia J. Clinical effectiveness of a class V flowable composite resin system. Compend Contin Educ Dent 1999; 20: 11-5.
- 4. Morigami M, Yukisada K, Tajima K, Sugizaki J, Uno S, Yamada T. A clinical survey on the frequency of flowable resin composite restorations. Adhes Dent 2011; 29: 55-9.
- 5. Anatavara S, Sitthiseripratip K, Senawongse P. Stress relieving behaviour of flowable composite liners: A finite element analysis. Dent Mater J 2016; 35: 369-78.
- 6. Ferracane JL. Resin composite –State of the art. Dent Mater 2011; 27: 29-38.
- 7. Karadas M. The effect of different beverages on the color and translucency of flowable composites. Scanning 2016; 38: 701-9.
- 8. Peutzfeldt A. Resin composites in dentistry: the monomer systems. Eur J Oral Sci 1997; 105: 97-116.
- 9. Ardu S, Duc O, Di Bella E, Krejci I. Color stability of recent composite resins. Odontology 2017; 105: 29-35.
- 10. Esmaeili B, Afkham S, Abolghasemzadeh F. The effect of time between curing and tea immersion on composite resin discoloration. Gen Dent 2018; 66: 64-8.
- 11. Duc O, Di Bella E, Krejci I, Betrisey E, Abdelaziz M, Ardu S. Staining susceptibility of resin composite materials. Am J Dent 2019; 32: 39-42.
- 12. Douglas WH, Craig RG. Resistance to extrinsic stains by hydrophobic composite resin systems. J Dent Res 1982; 61: 41-3.
- 13. Acar O, Yilmaz B, Altintas SH, Chandrasekaran I, Johnston WM. Color stainability of CAD/CAM and nanocomposite resin materials. J Prosthet Dent 2016; 115: 71-5.
- 14. Ferracane JL. Resin-based composite performance: Are there some things we can’t predict? Dent Mater 2013; 29: 51-8.
- 15. Ruyter IE, Nilner K, Möller B. Color stability of dental composite resin materials for crown and bridge veneers. Dent Mater 1987; 3: 246-51.
- 16. Kubo S. Longevity of resin composite restorations. Jpn Dent Sci Rev 2011; 47: 43-55.
- 17. Mjör IA, Morhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. Int Dent J 2000; 50: 361-6.
- 18. St Germain H, Swartz ML, Phillips RW, Moore BK, Roberts TA. Properties of microfilled composite resins as influenced by filler content. J Dent Res 1985; 64: 155-60.
- 19. Gajewski VES, Pfeifer CS, Fróes-Salgado NRG, Boaro LCC, Braga RR. Monomers used in resin composites: degree of conversion, mechanical properties and water sorption/solubility. Braz Dent J 2012; 23: 508-14.
- 20. Um CM, Ruyter IE. Staining of resin-based veneering materials with coffee and tea. Quintessence Int 1991; 22: 377-86.









