2022 年 22 巻 1 号 p. 5-11
2022 年 22 巻 1 号 p. 5-11
Purpose: The recent development of enamel-colored translucent zirconia makes it a viable material to produce crowns. However, to achieve a sufficient surface roughness for bonding, alumina particle abrasion must be employed. In this study, the effects of particle abrasion on the surface roughness of zirconia and the effects of sample pretreatment on the bond strength between zirconia and a composite resin core were investigated and compared to optimize the surface treatment conditions. Methods: Zirconia specimens were abraded with alumina particles and bonded to trial composite resin cores using a dual-cure composite luting agent and either an alumina-zirconia adhesive primer and/or a porcelain primer. The particle abraded zirconia surface was observed via laser scanning microscopy, and the roughness was evaluated qualitatively. Results: The peak-to-valley roughness, arithmetic mean height, root mean square height, and skewness parameter values significantly increased after particle abrasion. The bond strength was greater when the adhesive primer was applied to the zirconia specimens, and when the porcelain primer and a self-etching primer were applied to the composite resin cores, compared with specimens prepared without primers. With a self-etching primer, however, the porcelain primer played no role in increasing the bond strength. Conclusion: The combination of particle abrasion and a suitable primer is recommended to improve the bond strength between zirconia and the composite resin core. Moreover, the chemical surface treatment with a self-etching primer was more effective for adhesion than the silane coupling of the composite resin fillers.
Zirconia combines excellent mechanical properties [1] with good biocompatibility [2], and so it plays a major role in prosthetic treatments. Zirconia frames produced using computer-aided design (CAD)/computer-aided manufacturing (CAM) have been previously used in all-ceramic crowns with ceramic layering [3], and enamel-colored translucent zirconia with improved aesthetics has recently been developed [4,5,6,7], thereby making it possible to produce crowns using this material alone. Because such prostheses must adhere to the build-up of abutments, further studies on novel surface treatments for zirconia and its bonding with luting agent are needed. Indeed, a standardized cementation protocol that provides reliable results has yet to be established [8,9,10]. Improving the viability of zirconia prosthetic devices requires an appropriate surface treatment and the establishment of a cementation procedure [11]. Reliable cementation improves retention, prevents microleakage, and improves fatigue resistance in prosthetic devices [12].
Zirconia prosthetic devices produced using the CAD/CAM technique do not have sufficient surface roughness properties, which results in lower bond strengths [3]. Therefore, the zirconia surfaces must be roughened using methods such as alumina particle abrasion. Particle abrasion is a mechanical surface treatment process that improves the bond strength not only by increasing the adhesive interface area and surface roughness but also by removing organic contaminants from the zirconia surfaces, thereby increasing their wettability [13,14,15,16]. One advantage of particle abrasion is that it can be easily performed at the chairside or in a dental laboratory. However, it has been suggested that particle abrasion with alumina may cause surface damage that affects prosthetic survival rates in repeated fatigue tests [17,18]. Therefore, particle abrasion must be performed under conditions that do not damage the zirconia surface. This can potentially be achieved using primers for chemical surface treatment. For example, alumina-zirconia adhesive primer (AZ) is recommended for zirconia, whereas porcelain primer (PP) is typically used for silica.
In this study, the effects of particle abrasion on the surface roughness of zirconia were examined based on five surface roughness parameters. More specifically, the correlation between the surface roughness parameters and shear bond strength of zirconia after particle abrasion were investigated. In addition, the effect of different primer pretreatments on the bond strength was compared using shear bond strength tests. The null hypothesis of this study was that pretreatment with different primers does not affect the shear bond strength.
Cylindrical pieces (10 mm diameter, 30 mm height) of yttria-stabilized zirconia (Tosoh, Tokyo, Japan), with the composition Y2O3 (5.15 wt%), Al2O3 (0.25 wt%), SiO2 (≤0.02), Fe2O3 (≤0.01), Na2O (≤0.04), and ZrO2 + HfO2 + Y2O3 + Al2O3 (≥99.9 wt%), as listed in Table 1, were used as test specimens. The firing schedule included heating (100˚C/h), holding (1,500˚C for 2 h), and cooling (natural cooling). For polishing, pre-grinding was performed with the use of a #200 diamond wheel (Keihin Kogyosho, Tokyo, Japan). A spiral copper plate (Keihin Kogyosho) and a 3 μm diamond abrasive (Keihin Kogyosho) were used for rough polishing and the removal of grinding marks. For finish polishing, a buff and 3 μm diamond abrasive (Keihin Kogyosho) was employed.
Table 1 Material employed in this study
| Code | Material | Composition | Manufacturer |
|---|---|---|---|
| RC | ResiCem | Paste A: UDMA, TEGDMA, fluoro-alumino-silicate glass, others | Shofu, Kyoto, Japan |
| Paste B: UDMA, TEGDMA, fluoro-alumino-silicate glass, 4-AET, HEMA, others | |||
| AB | ResiCem Primer | Primer A: purified water, acetone, reaction initiator, others | |
| Primer B: 2-HEMA, 4-AET, acetone, others | |||
| AZ | AZ Primer | Acetone, 6-methacryloxyhexylphosphonoacetate, others | |
| PP | Porcelain Primer | Ethanol, silane coupling agent, others | |
| Zirconia | Yttria-stabilized zirconia | Y2O3, Al2O3, SiO2, Fe2O3, Na2O, ZrO2, HfO2 | Tosoh, Tokyo, Japan |
4-AET: 4-acryloxyethyl trimellitate, HEMA: 2-hydroxyethyl methacrylate, TEGDMA: triethyleneglycol dimethacrylate, UDMA: urethane dimethacrylate
The surfaces of the zirconia specimens before and after particle abrasion were observed by laser scanning microscopy (VK-100, Keyence, Osaka, Japan) using an objective lens (20×). Scanning was performed in the height display and laser image modes. The zirconia was placed on the microscope stage, and the surface was scanned using a 0.2 μm spot-size laser beam. Five surface roughness parameters were measured seven times, including the peak-to-valley roughness (Rz), arithmetic mean height (Ra), root mean square height (Rq), skewness (Rsk), and kurtosis (Rku) (Table 2).
Table 2 Surface roughness parameters analyzed, including their classifications, symbols, names, and definitions
| Symbol* | Name | Definition |
|---|---|---|
| Rz | Peak-to-valley roughness | The sum of the maximum peak height value and the maximum pit depth value |
| Ra | Arithmetic mean height | The arithmetical mean of the absolute values of height |
| Rq | Root mean square height | Root mean square at the reference length, which relates to the standard deviation of the surface roughness |
| Rsk | Skewness | The degrees of profile skew, which expresses the symmetry of peaks and valleys using the average line as the center |
| Rku | Kurtosis | A measure of the sharpness of the roughness profile. The higher the value, the sharper the height profile |
*ISO 13565-1, Amplitude/height parameters
The composite resin cores were prepared by coating a cylindrical acrylic ring (3 mm inner diameter, 3 mm height) with the separation material (COE-SEP, Shofu, Kyoto, Japan), pouring in composite resin (BeautiCore LC, Dentin, Shofu), pressing with a glass plate, photo-irradiating for 30 s with a light-curing device (Solidilite, Halogen lamp JCR 110V 150 W/s, Shofu), and polishing with #1000 water-resistant polishing paper after photopolymerization.
The zirconia was particle abraded as commonly carried out in clinical settings using an abrader (Pencil Jet, Yoshida, Tokyo, Japan) with alumina particles (50 μm) at 0.2 MPa and blasting from a distance of 10 mm. The tip of the abrader was set at 90˚ from the zirconia surface. After particle abrasion, the samples were subjected to ultrasonically activated irrigation in distilled water and dried. The particle abraded zirconia was abbreviated as SB zirconia.
Composite luting agent and primersThe luting agent and primers used in this study are listed in Table 1. A dual-cure composite luting agent (ResiCem, Shofu, RC) was used. For surface preparation, a single liquid silane primer (Porcelain Primer, Shofu, PP), a self-etching primer (ResiCem Primer, Shofu, AB), and an acidic primer (AZ Primer, Shofu, AZ) were employed. The RC luting agent was cured by photoirradiation for 20 s using the Penbright unit (1,200 mW/cm2, Shofu). Before curing the RC, the composite resin was pressed from the top using a 9.8 N force that was maintained during the curing process. Further, the cement cover was removed before complete curing. After 1 day, shear bond strength was determined.
Shear bond strength testThe shear bond strength tests were performed using a universal testing machine (EZ-Test, Shimadzu, Kyoto, Japan) at a crosshead speed of 1 mm/min; each test was repeated seven times. The shear bond strength (MPa) was calculated by measuring the maximum load (N) at which the bonded part of the specimen failed, and this value was divided by the bonded area.
The bonding conditions are listed in Table 3. In the condition 1, no primers were used on the SB zirconia. In the condition 2, AZ Primer was applied to the SB zirconia, and Porcelain Primer was applied to the composite resin. In the condition 3, AZ Primer was applied to the SB zirconia, and ResiCem Primer was applied to the composite resin. In condition 4, AZ was applied to the SB zirconia, and ResiCem Primer was applied to the composite resin following the application of Porcelain Primer. The shear bond strength was measured seven times in all four bonding conditions tested herein.
Table 3 Bonding conditions accessed
| Zirconia | Composite resin core material | ||
|---|---|---|---|
| Priming* | AZ | PP | AB |
| Condition 1 | - | - | - |
| Condition 2 | 〇 | 〇 | - |
| Condition 3 | 〇 | - | 〇 |
| Condition 4 | 〇 | 〇 | 〇 |
*AZ: AZ primer, PP: Porcelain primer, AB: ResiCem Primer A + B
One-way analysis of variance was used to test for significant differences in the surface roughness (Rz, Ra, Rq, Rsk, and Rku) before and after particle abrasion of zirconia. Data are expressed as group means ± standard deviations. The results with a probability level of ≤0.01 were considered significant.
One-way analysis of variance was also used to test for significant differences in the bond strength between zirconia and the composite resin subject to the four bonding conditions tested herein. The Bonferroni test for multiple comparisons was performed as a post-hoc test. Normality (Shapiro-Wilk test) and homoscedasticity (Bartlett's test) were evaluated prior to statistical analysis. All data are expressed as group means ± standard deviations. Probability levels ≤0.01 were considered statistically significant. The ESUMI Excel statistics software (version 7.0, ESUMI Corp., Tokyo, Japan) was used for statistical processing. In addition, a correlation analysis between the surface roughness and the shear bond strength after particle abrasion was performed.
Figure 1 shows the zirconia surface profiles before and after particle abrasion. The height display mode shows the surface height distribution in color, and the laser image mode shows the surface profile. Linear marks were observed before particle abrasion, which appeared in regular, parallel stripes of peaks and valleys. After particle abrasion, the stripe-shaped marks generated by polishing disappeared and were replaced by fine irregularities over the entire surface.
Zirconia surface roughness before and after particle abrasionFigure 2 shows the changes in surface roughness caused by particle abrasion. The peak-to-valley roughness (Rz; 33.92 ± 11.93 μm), arithmetic mean height (Ra; 1.76 ± 0.04 μm), root mean square height (Rq; 2.22 ± 0.06 μm), and skewness (Rsk; −0.21 ± 0.02) were significantly larger after particle abrasion) than before particle abrasion (Rz: 15.74 ± 2.22 μm; Ra: 0.91 ± 0.02 μm; Rq: 1.19 ± 0.01 μm; Rsk: (−0.65 ± 0.12). In contrast, the kurtosis, Rku, was significantly smaller after particle abrasion (3.28 ± 0.07) than before particle abrasion (4.09 ± 0.20).

Observation of the surface morphology before and after particle abrasion, as observed using a shape analysis laser microscope
Panels (a) and (b) show the surface height display while (c) and (d) show the surface profiles. Panels (a) and (c) show the surface before particle abrasion while (b) and (d) show the surface after particle abrasion.

Fig. 2 Changes in the zirconia surface roughness as a result of particle abrasion
The surface roughness parameters are as follows: peak-to-valley roughness (Rz), arithmetic mean height (Ra), root mean square (RMS) height (Rq), skewness (Rsk), and kurtosis (Rku) (* P < 0.01).

Mean shear bond strengths of the luting agented to zirconia and a composite resin core material
Data are presented as means ± standard deviations (* P < 0.01).
Shear bond strength testIn the normality (Shapiro-Wilk test), condition 1 has a P -value of 0.536, condition 2 has a P -value of 0.463, condition 3 had a P -value of 0.011 and condition 4 had a P -value of 0.835. The homoscedasticity (Bartlett's test) had a P -value of 0.542. Figure 3 shows the results of the shear bond strength test obtained at the four test conditions employed in this study. As indicated, the shear bond strength following treatment with condition 1 (11.7 ± 1.8 MPa) was almost half that following condition 2 (23.7 ± 4.7 MPa), while condition 3 (33.8 ± 3.1 MPa) and condition 4 (34.1 ± 7.1 MPa) presented significantly higher ( P < 0.01) bond strengths than conditions 1 and 2. Between conditions 3 and 4, no significant difference was observed.
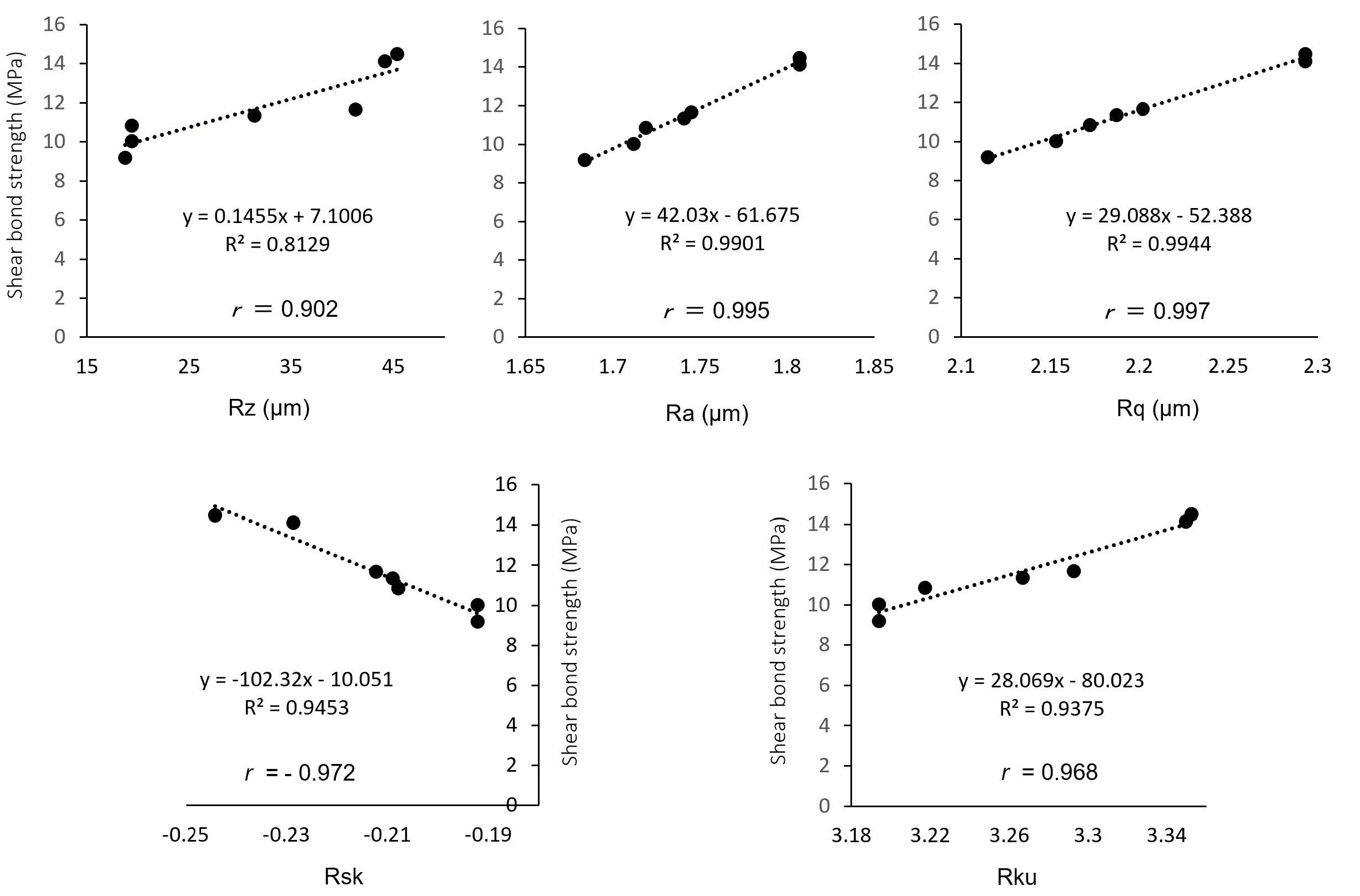
Fig. 4 Linear equations and correlation coefficients between the shear bond stress and surface roughness parameters
The surface roughness parameters are as follows: peak-to-valley roughness (Rz), arithmetic mean height (Ra), root mean square height (Rq), skewness (Rsk), and kurtosis (Rku).
Correlation between the surface roughness and the shear bond strengthThe correlations between the shear bond strength after particle abrasion and the surface roughness parameters (Rz, Ra, Rq, Rsk, and Rku) are shown in Fig. 4, where all surface roughness parameters exhibited significant correlations with the shear bond strength. The correlation coefficients for Rz, Ra, Rq, Rsk, and Rku were 0.902, 0.995, 0.997, −0.972, and 0.968, respectively.
The shear bond strengths obtained following treatment with conditions 3 and 4 were approximately three times larger than those obtained using condition 1. In a preliminary experiment in which zirconia was used without particle abrasion (instead of SB zirconia), AZ plus any primer did not produce a sufficient bond strength to withstand the measurement. This finding led us to perform shear bond strength measurements with SB zirconia.
As mentioned above, particle abrasion is a process in which alumina particles are blown onto a surface at high pressures. Spraying alumina particles onto zirconia roughens the surface and improves its wettability [19]. However, particle abrasion may also cause surface damage, defects, and cracks that may compromise the mechanical properties of zirconia [20]. Therefore, particle abrasion must be performed with suitable pressure, distance, and particle size settings. Souza [21], therefore, recommended performing particle abrasion with the use of 30 µm alumina particles at 0.25 MPa to avoid damaging the zirconia, while Özcan [22] proposed particle abrasion using alumina particles (diameter 30-50 µm) at 0.05-0.25 MPa for at least 20 s. In this study, particle abrasion was performed using 50 μm alumina particles at 0.2 MPa.
The laser scanning microscopy images recorded after particle abrasion showed that the stripe-shaped polishing marks observed before particle abrasion had disappeared and changed to fine irregularities over the entire surface. In addition, Rz, Ra, and Rq approximately doubled, and Rsk approximately tripled after particle abrasion. This increase in surface roughness was expected to improve the micromechanical interlocking of the RC to the zirconia surface. However, the bond strength obtained following the treatment with condition 1, in which the surface was modified solely by the mechanical surface treatment of particle abrasion, was only approximately one-third of the bond strength obtained using condition 4. This finding indicates that the bonding of zirconia not only requires mechanical surface treatment by particle abrasion but also an appropriate chemical surface treatment.
As zirconia does not contain silicon, silane coupling agents that are effective for porcelain cannot be utilized. Therefore, AZ was applied to the zirconia surfaces in this study. AZ increases adhesion via the phosphonate group of 6-methacryloyloxyhexyl phosphonoacetate, which acts on the hydroxyl groups on the zirconia surface [4]. In addition, PP increases adhesion through a silane coupling agent that reacts with the OH-Si moieties present in the CRs that are used as inorganic fillers [4]. The finding that the bond strength was approximately 1.6 times higher when AB was applied to the CRs than when PP was applied suggests that AB is more effective in bonding to the RC and/or strengthening the RC. The latter possibility may be consistent with the report by Kamada et al. [4] who showed that AB accelerated the curing of RC.
Another minimal approach regarding the repair of resin composite restorations without silane coupling pretreatment has also been reported [23]. Furthermore, Akimoto et al. [24] reported that silane coupling treatment is not necessary for the adhesion of resin composites, depending on the composition of the fillers, owing to the chelating reaction between the acidic monomer and the Sr and Ba alkaline-earth metal ions. AB (a self-etching primer) contains an acidic monomer, while the CRs (BeautiCore LC) contain a S-PRG filler, which itself contains strontium as a glass component. The acidic monomer of the primer, therefore, adheres to strontium to undergo a chelation reaction.
Thus, the promotion of RC curing by AB improves the bond strength to a greater extent than when using silane coupling. In other words, the promotion of curing by AB, which comprises 2-hydroxyethyl methacrylate (2-HEMA) and 4-acryloxyethyl trimellitate (4-AET), may be more advantageous for bonding than the silane coupling of CR fillers.
Condition 4, in which AB was applied after PP, resulted in the same bond strength as condition 3 (i.e., AB alone), thereby indicating the lack of synergistic effects between the two primers. The shear bond strengths resulting from treatment with conditions 3 and 4 were obtained using different primers. The conditions 3 and 4 suggest the existence of a plateau of the bond strength of the composite RC itself. This plateau may explain why there was no difference in the shear bond strength between conditions 3 and 4.
The values of Rz, Ra, Rq, Rsk, and Rku were then measured. Each surface roughness parameter was positively correlated to the shear bond strength after particle abrasion. Of the five surface roughness parameters examined, Ra and Rq exhibited the largest correlation of r ≥ 0.995, thereby indicating that these parameters exert the greatest effect on the final bond strength. In this study, particle abrasion was performed at 0.2 MPa due to clinical recommendations. In future studies, we will particle abrasion zirconia at two or three different pressures to determine the optimal relationship between the surface roughness and the bond strength.
Finally, it is notable that this study has limitations regarding its ability to simulate changes in the oral environment and the clinical loading forces impaired on the restorations. Furthermore, the temperature and moisture of the oral cavity were not simulated, and the specimens were not thermally cycled. In future studies, ageing parameters should be included to explore the viability of this surface treatment.
In conclusion, mechanical surface treatment with particle abrasion modifies the zirconia surface layer to create fine irregularities that improve mechanical interlocking. For the chemical surface treatment, AB (which comprised 2-HEMA and 4-AET) was more effective for adhesion than the silane coupling of the CR fillers. The bond strength was found to be approximately three times greater when AZ was applied to the zirconia specimens and when PP and AB were applied to the CRs compared with the case in which no primers were applied. Thus, the combination of particle abrasion with a suitable primer is recommended to improve the bond strength between zirconia and a CR.
Conflict of Interest
The authors declare that they have no conflict of interest.