2021 年 129 巻 2 号 p. 121-132
2021 年 129 巻 2 号 p. 121-132
Maize (Zea mays) was an important staple and ceremonial food in the pre-Columbian Andean world. Previous researchers have studied maize agriculture in early ancient Andean society by examining macro- and microbotanical remains. However, isotope analyses of human remains have shown that maize was not a primary food resource during the Formative Period (1800–1 cal BC). Although a few studies have suggested that maize was consumed in this period, we know little about how the dietary role of maize differed across the Andean society and how it changed over time. This study measures carbon and nitrogen isotope ratios from human and animal bone collagen samples and human tooth enamel samples excavated from the Pacopampa archaeological site in the highlands of northern Peru in order to better understand maize consumption in this period. The site dates to the Middle to Late Formative Periods (1200–700 cal BC, 700–400 cal BC) and the Early Cajamarca phase (cal AD 200–450). The findings indicate an increase in C4 resource consumption during the Late Formative Period—an increase that we attribute directly to maize and indirectly to domesticated animals. Although dietary variation related to social stratification was insignificant at this site, it has been reported at and between some coeval sites. Thus, we conclude that when these populations began exploiting C4 resources, their strategic use of these resources varied depending on the site. This study suggests that the use of maize during the Formative Period was probably greater and earlier than reported in previous isotope studies. In addition, maize utilization for domestic animals in this period, which has rarely been mentioned, was also important.
Maize (Zea mays) was one of the most important staple foods in pre-colonial South America. Colonial writers have documented various maize preparations from the 16th to 17th centuries, such as roasted maize, boiled maize, maize dumplings, toasted or steamed maize bread, and popcorn (Garcilaso de la Vega, 2006 [1609]: 67–69; Rowe, 1946: 220). Chroniclers also recorded that the Inca, the last precolonial society, utilized maize as a sacred food. Indeed, the fact that it was a symbolically significant food in Inca mythology (Betanzos, 1880 [1551]: 16; Murra, 1973: 398; Molina, 2011 [1575]: 11; Morris and von Hagen, 2011: 26) may have promoted maize production. Inca emperors practiced ceremonial agriculture and drank large amounts of maize beer (chicha) at various ceremonial events (Staller, 2006). Considering the importance of maize to life and culture during the Inca period (from the 15th century to AD 1532), researchers have taken an interest in the economic and ritual contributions of maize in embryonic Andean societies and the ways in which these contributions cohered with ceremonial architecture (Seki, 1998; Shady, 2006; Staller, 2006; Logan et al., 2012).
It is generally thought that maize has been consumed since the Archaic and Formative Periods based on botanical evidence. Maize was originally domesticated in Central America (Matsuoka et al., 2002; Piperno et al., 2009; Ranere et al., 2009), and spread to North and South America (Blake, 2006; Bonzani and Oyuela-Caycedo, 2006; Gil et al., 2006; Bonavia, 2013). Maize remains have been reported in various Andean sites of the Archaic Period (c. 5000–3000 BC) and the Initial Formative Period (c. 3000–1800 BC), especially in dry coastal areas (e.g. Shady, 2006; Grobman et al., 2012; Bonavia, 2013). Not only were macrobotanical remains such as maize cobs, grains, and husks excavated, but also starch granules and phytoliths, which were recovered from lithic tools and vessels (Zarrillo et al., 2008; Haas et al., 2013) and human and animal coprolites (Bonavia, 2013; Haas et al., 2013). These studies confirm that maize was produced, processed, and consumed in this region for thousands of years.
Macro- and microbotanical maize remains have been difficult to find in the Peruvian highlands because of the high rates of precipitation there—for instance, no macrobotanical maize remains were found in excavations at Kotosh and Huacaloma (Matsutani, 1972; Terada and Onuki, 1982). Moreover, the lack of surplus food storage in this region and isotopic analyses of human remains in the highlands suggest that there was no large-scale maize production in the Peruvian highlands during the Initial and Early Formative Periods (Seki, 1998; Seki and Yoneda, 2005). However, this does not imply that there was no small-scale agriculture in the region at this time; some studies have reported maize utilization at highland sites, including maize starch granules and phytoliths (Perry et al., 2006; Logan et al., 2012; Clasby, 2019), and maize pollen (Chepstow-Lusty, 2011). These findings have forced us to reconsider the scale of maize agriculture in the Peruvian highlands during this period and the ways in which it changed over time. The question this study asks is: what role did maize play in food resources? As it is difficult to determine the proportion of maize in these peoples’ diets from macro- and microbotanical remains alone, we examined carbon and nitrogen ratios in bone collagen and tooth enamel samples to estimate the protein and energy contribution of maize at individual levels.
Carbon and nitrogen isotope ratios (δ13C and δ15N) have been widely used for dietary estimations in Andean studies (Verano and DeNiro, 1993; Burger et al., 2003; Tomczak, 2003; Finucane et al., 2006; Knudson et al., 2007; Williams and Katzenberg, 2012). The analyses in these studies are based on differences in the isotope ratios of different types of food. For example, C4 plants have higher carbon isotope ratios than C3 plants because they enact photosynthesis in different ways (Park and Epstein, 1960; Smith and Epstein, 1971; O’Leary, 1981). Most cultivated plants in the Andean region are C3 plants, which have low δ13C values—by contrast, both maize and amaranth (Amaranthus caudatus, also known as kiwicha) are the only C4 plants cultivated in the region that have high δ13C values. Furthermore, legumes are C3 plants with low δ15N values in general because of the nitrogen-fixing bacteria in their roots (Mariotti, 1983). With this information in mind, we can describe ancient peoples’ and animals’ diets with some precision. For instance, we know that the body tissues of terrestrial herbivores reflect the isotopic signature of the plants they consume, and so we can reconstruct their diets by observing how the meat of C4 plant consumers possesses high δ13C values and the meat of C3 plant consumers possesses low δ13C values. We also know that seafood (e.g. fish, shellfish, and marine mammals) possess high δ13C and δ15N values because heavy isotopes are stored and concentrated in their bodies via the food chain (Schoeninger et al., 1983; Schoeninger and DeNiro, 1984). In this paper, we use the term ‘C4 resources’ to describe both cultivated C4 plants and meat from animals that fed mostly on C4 plants.
The stable carbon and nitrogen isotope ratios in animal tissues depends on the diet and type of nutrient. In herbivores, food protein is utilized for the growth and maintenance of protein tissues such as collagen, while carbohydrates and fats are used for energy metabolism and tissue synthesis (Ambrose and Norr, 1993). As hydroxyapatite, the primary inorganic constituent of bone and tooth enamel, is formed from blood bicarbonate derived from energy metabolism, the carbon isotope ratios of bone carbonate (δ13Cap) and tooth enamel (δ13Cen) reflect the total dietary carbon, including carbohydrates and fats. In carnivores, fats and proteins contribute to energy metabolism, although collagen is also formed from protein resources. Humans generally eat a varied diet, and while the isotope ratios of bone collagen (δ13Ccol) also strongly relate to the isotope ratios of consumed food proteins, those of bioapatite correlate with the whole diet (Ambrose and Norr, 1993; Tieszen and Fagra, 1993).
We should note here that different tissues can provide different dietary records because they have differential turnover rates. For example, bone collagen of human adults provides a dietary record at a decadal level before death (Babraj et al., 2005; Hedges et al., 2007). Furthermore, enamel formation differs by tooth type and the isotopic information of each tooth varies with temporal changes in diet. For example, the third molar can be used to determine dietary isotope signature between ages 9 and 13 in humans, and the first and second molars can be used to examine childhood diets, including the effects of breastfeeding (Hilson, 1996; Fuller et al., 2006). It is possible to consider the ancient diet in more detail by using the difference in the metabolism of these tissues.
C4 resource consumption during the Formative PeriodSeveral studies that have estimated ancient Andean peoples’ diets in this period via isotope ratios in bone collagen have revealed that these people consumed low amounts of C4 resources during the Formative Period (Figure 1, Figure 2) (Burger and van der Merwe, 1990; Tomczak, 2001; Coutts et al., 2011). This finding is unexpected because botanic evidence suggests that maize was utilized during this period. These reports showed that Andean peoples consumed a mixed diet of C3 and marine resources in coastal areas and consumed simple diets that were heavily dependent on C3 resources in highland areas. This suggests that food resource strategies during the Formative Period were strongly related to the surrounding environment.
Map of sites with stable isotope data available for the Formative Period.
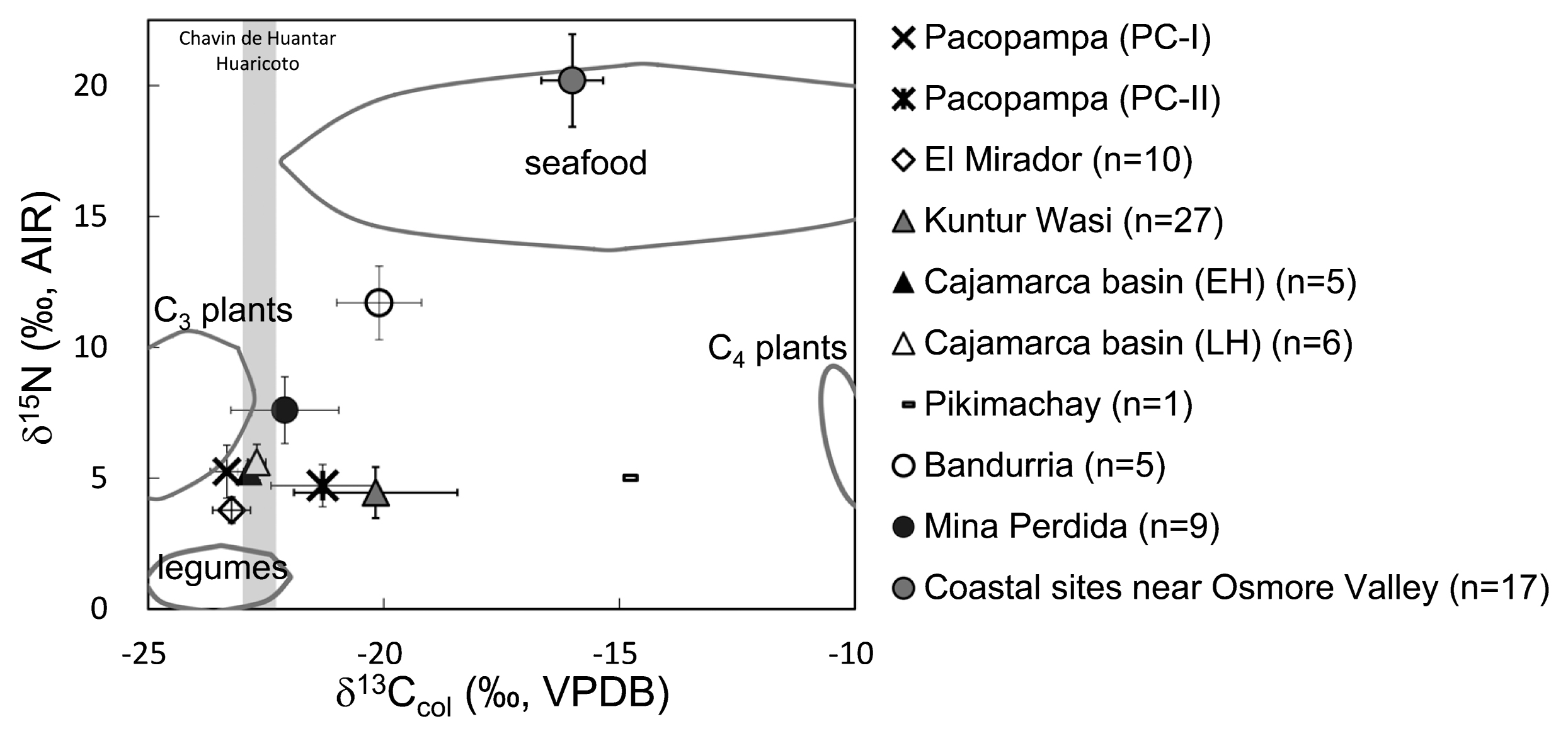
Summary of previous isotope studies using bone collagen in the Formative Period (Burger and van der Merwe, 1990; Coutts et al., 2011; Finucane 2009; Seki and Yoneda, 2004; Tomczak, 2001; Tykot et al., 2006). Because nitrogen isotope ratios of Chavin de Huantar (n = 5, −18.7 ± 0.4‰) and Huaricoto (n = 3, −18.6 ± 0.4‰) have not been reported, the carbon isotope ratios are represented as a vertical gray band. Pacopampa site data are reported in this study. Ellipses drawn with gray lines show isotope values for food resources in the Andean region. They are Mahalanobis ellipses (1σ) estimated with isotope data from previous studies (DeNiro, 1988; DeNiro and Hastorf, 1985; Falabella et al., 2007; Szpak et al., 2013; Tieszen and Chapman, 1992; Turner et al., 2010; Verano and DeNiro, 1993). Plotted data are the isotope fractionation corrected by 4‰ for δ13Ccol and 3‰ for δ15N.
However, evidence found at the Pikimachay and Kuntur Wasi archaeological sites suggests that C4 resources were indeed consumed during the Formative Period. Of these two sites, the Pikimachay site has more extensive carbon isotope data (Finucane, 2009). However, because studies of the Pikimachay site have only analyzed data from one individual from the Formative Period, they do not provide substantial evidence that high C4 resource consumption was typical at that site. By contrast, isotope analyses of 27 individuals from the Kuntur Wasi site indicated that these people certainly consumed C4 resources (Seki and Yoneda, 2004, 2005).
Few investigations of temporal dietary changes during the Formative Period have been conducted in the Andes. In the coastal regions, maize cultivation increased in the era following the Formative Period (Ericson et al., 1989; Kellner and Schoeninger, 2012; Lambert et al., 2012). Regarding mountainous regions, an increase in C4 resource consumption was observed between the Middle and Late Formative Periods in the Cajamarca region (Seki and Yoneda, 2004, 2005). However, these latter studies did not exclude the possibility of microregional variations in diet. Because the Kuntur Wasi site contains very few human remains from the Middle Formative Period (or Idolo phase) (1200–800 cal BC), they analyzed human bone samples from the Early and Late Huacaloma phases (1500–1000 cal BC and 1000–550 cal BC) (Seki and Yoneda, 2004) in the Cajamarca Basin. The site is located 40 km from the Cajamarca Basin and is at a relatively low altitude. In other words, the difference in isotope ratios may not indicate dietary changes between periods, but might rather reflect dietary diversity across different ecological environments.
Regarding the analysis of carbon isotope ratios of bioapatite (δ13Cap), there are only two reports on the Formative Period: Badurria at the coastal area and El Mirador in the highland area. Marine resources had been the primary food at the Badurria site (Coutts et al., 2011). On the other hand, at the El Mirador site, the δ13Cap values of bone apatite suggested greater C4 plant consumption, although low δ13Ccol values were detected (Tykot et al., 2006). Furthermore, remains at the Tablada de Lurin site dating to the Final Formative Period and Early Intermediate Period (200 BC–AD 200) (Tykot et al., 2006) have been found to have higher δ13Ccol, δ15N, and δ13Cap values than remains found at El Mirador, which indicates that people living at the Tablada de Lurin site consumed both C4 resources and marine resources.
These previous studies of isotope ratios have suggested that C4 resource consumption varied by region, and therefore that maize cultivation was introduced at different times in different regions. We suggest that inter- and intra-site diversification of subsistence strategies may reflect the timing of initial maize utilization. Macrobotanical maize remains and evidence of camelid herding in the Cajamarca region dated to the Late and Final Formative Periods (Terada and Onuki, 1985: 267–273) might imply the emergence of social stratification and changes in peoples’ subsistence systems across time and space. However, as noted in our previous study (Takigami et al., 2020), camelid husbandry using C4 plants began in the Late Formative Period; therefore, we assume that this study will reveal that C4 resource exploitation in this region changed over time during the course of the Formative Period. We aim to investigate maize cultivation during the Formative Period by analyzing samples of human and animal bones and human tooth enamel. Temporal dietary changes were investigated on the basis of δ13Ccol and δ15N values, and dietary differences related to social stratification were estimated from δ13Ccol and δ13Cen values.
The Pacopampa site is located at an elevation of 2500 m in the district of Querocoto, Chota Province, Cajamarca region, in the northern highlands of Peru (Figure 1). It is a large ceremonial site developed during the Middle and Late Formative Periods, and constructed using three large platforms on a mountaintop. The platform on the top terrace was the largest, and the site’s main structures were concentrated on this platform (Seki, 2014). The samples used in this study were excavated from this platform. A recent investigation by the Pacopampa Archaeological Project (organized by the Universidad Nacional Mayor de San Marcos, Peru, and the National University of Mayor de San Marcos, Peru, and the National Museum of Ethnology, Japan) revealed that the site was established in two phases: Pacopampa I (PC-I: 1200–700 cal BC) and Pacopampa II (PC-II: 700–400 cal BC) (Seki et al., 2019). Thus, the PC-I phase corresponds to the Middle Formative Period, and the PC-II phase corresponds to the early Late Formative Period.
Sampling strategyWe collected 55 bone samples and 8 tooth enamel samples from 55 human individuals in the site. Nine individuals dating to PC-I were scattered bones, except for 11PC-CEnt. 1-H1 which is an individual burial. Four individuals date to the Early Cajamarca phase (cal. AD 200–450) and the other 42 individuals date to PC-II. Most PC-II individuals were found in single burials. The 09PC-C-Ent.09-2-H2 and 12PC-B2-Ent.530 individuals were found buried with gold ornaments, and the 12PC-B-Ent.532-H1 individual was buried with a silver needle as a funerary offering. Tooth samples were from individually buried human remains in PC-II phase. Tooth enamel samples were collected from the third molar (except in the case of 09PC-B2-Ent 508-6, from which we collected enamel samples from the second molar).
We also analyzed 60 faunal bones, including 19 deer (Odocoileus virginianus) individuals, 24 camelid (Camelidae) individuals, 16 guinea pig (Cavia porcellus) individuals, and 1 dog (Canis familiaris) individual to compare with the human diet. As camelids and guinea pigs are domesticated animals, we expect to find that their isotope ratios indicate that their feeding was managed by humans. We collected faunal bones from both the PC-I and PC-II phases and the dog was from the PC-I phase. Camelid data from PC-I were reported in our previous study (Takigami et al., 2020).
MethodsThe bone samples were treated using an improved acid–alkali treatment (Yoneda et al., 2002) and our collagen extraction process was based on Longin’s (1971) method. After physically removing the soil, we soaked the samples in 0.2 M NaOH to remove organic soil contaminants such as humic acid and fulvic acid. The samples were then dried and powdered. The powdered samples were then transferred to cellulose tubes and reacted with 1.2 M HCl to dissolve and remove inorganic matter. To extract gelatin collagen, purified water was added to the organic residues, which were then heated to 90°C for 12 hours and filtered by using vacuum filtration. Finally, the filtered solution was freeze-dried in order to obtain solid gelatin and collagen.
We conducted measurements at the National Institute for Environmental Studies, Tsukuba, Japan, and the Laboratory of Radiocarbon Dating, The University Museum, The University of Tokyo, Japan. Measurements were conducted using an elemental analyzer coupled to an isotope ratio mass spectrometer (Carlo Erba NA1500, Carlo Erba Instruments, Milano, Italy; Finnigan MAT ConFlo II interface, and Finnigan MAT 252, Flash 2000, Finnigan ConFlo VI interface, and Delta V Advantage, Thermo Fisher Scientific, Bremen, Germany). The standard deviations estimated from running standards were 0.1‰ and 0.2‰ for δ13C and δ15N, respectively. We estimated the contamination and diagenetic alteration of collagen via the C:N atomic ratios. As the C:N atomic ratios of unaltered collagen extracted from modern animal bones fall within the range of 2.9–3.6, we assumed that the ancient collagen samples whose C:N ratios fell outside this range may have been contaminated or altered by diagenesis (DeNiro, 1985) and excluded these samples from further analysis.
We chemically treated our tooth samples in the way described above, following Balasse et al. (2002) and Kusaka and Nakano (2014). After polishing the surfaces of our tooth samples, we collected approximately 15 mg of tooth enamel using a dental drill. The powdered enamel was soaked in 2.5% sodium hypochlorous acid for 12 hours to remove organic matter. The samples were then rinsed with Milli-Q water and reacted with 0.1 M buffered acetic acid for 30 minutes to clean the surface of the powdered enamel. After pretreatment, δ13Cen was measured with a Kiel IV carbonate device coupled to an isotope ratio mass spectrometer (Kiel IV and MAT 253, Thermo Fisher Scientific, Bremen, Germany) at the National Museum of Nature and Science, Tsukuba, Japan. We estimated the standard deviation of δ13Cen at 0.02‰ from an international standard (NBS-19). We then calculated statistical significance by the Mann–Whitney U test with SPSS 22 statistical software (IBM). The isotope fractionations between bone collagen and diet are 3–5‰ in carbon isotope ratio and 2–4‰ in nitrogen isotope ratio (Ambrose, 2000; Ambrose and Norr, 1993; DeNiro and Epstein, 1978, 1981; Lee-Thorp et al., 1989; Schoeninger and DeNiro, 1984; Tieszen and Fagre, 1993). In order to compare with isotope data of food, we corrected the isotope fractionation by 4‰ for δ13C and 3‰ for δ15N in the figures.
The C:N ratios for all animal collagens were within the acceptable range mentioned above (Table S1). The contents of our camelid samples indicated that they underwent dramatic dietary changes between the Middle and Late Formative periods—by contrast, the diets of our deer and guinea pig samples remained constant (Figure 3). The average values with one standard deviation for PC-I and PC-II deer were −18.7 ± 1.6‰ and −18.9 ± 1.3‰ for δ13Ccol, and 5.1 ± 1.0‰ and 6.0 ± 0.8‰ for δ15N, respectively (Table 1). Thus, there was a significant difference in δ15N values between PC-I and PC-II, and no significant differences in δ13Ccol values (Mann–Whitney U test: P = 0.842 for δ13Ccol and P = 0.028 for δ15N). The isotope data for deer indicated that C3 plants were a large part of their diet. The guinea pig samples possessed higher δ13Ccol values than deer samples: −15.2 ± 3.9‰ and −15.1 ± 2.8‰ for δ13Ccol and 3.5 ± 1.5‰ and 5.1 ± 1.3‰ for δ15N in PC-I and PC-II, respectively. We only found significant differences in the δ15N values between the two periods (Mann–Whitney U test: P = 0.878 for δ13Ccol and P = 0.050 for δ15N). Changes in deer and guinea pig δ15N values may reflect changes in their environment—for example, aridification (which elevates δ15N values) seems likely because Pacopampa is located on the upper eastern slopes of the Andes, and the site is susceptible to the Amazonian climate. Furthermore, the δ13Ccol values revealed that our guinea pig samples consumed C4 plants in both PC-I and PC-II.
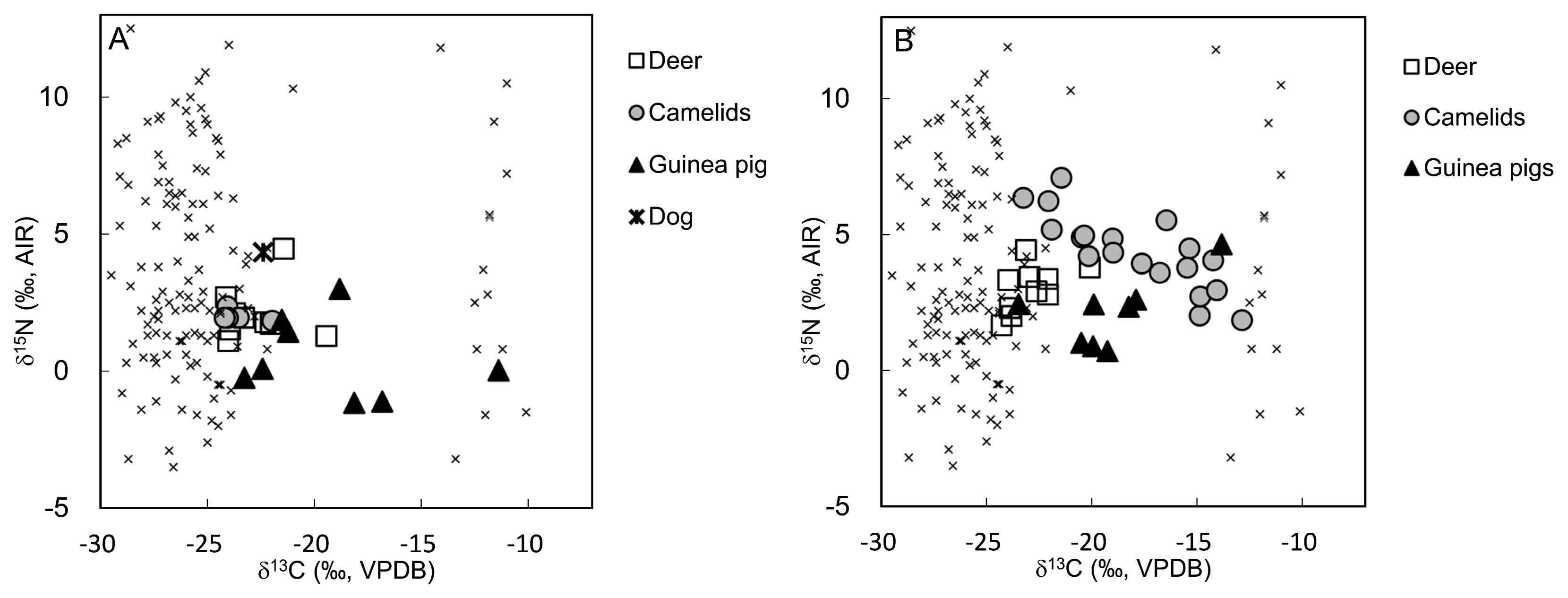
Carbon and nitrogen isotope ratios of animal bones from the Pacopampa site. (A) PC-I phase, B: PC-II phase. Cross marks indicate modern wild plants (Szpak et al., 2013). The influence of the Suess effect on carbon isotope ratios was corrected by 1.5‰ (Friedli et al., 1986; Keeling, 1979).
| Phase | N | δ13Ccol (‰) | δ15N (‰) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Min. | Max. | Mean | SD | Median | Min. | Max. | |||
| Animal (Pacopampa site) | ||||||||||||
| Deer | PC-I | 9 | −18.7 | 1.6 | −19.3 | −20.1 | −15.4 | 5.1 | 1.0 | 4.8 | 4.1 | 7.5 |
| Deer | PC-II | 10 | −18.9 | 1.3 | −19.1 | −20.3 | −16.1 | 6.0 | 0.8 | 6.1 | 4.7 | 7.4 |
| ave. | −18.8 | 1.4 | −19.1 | 5.6 | 1.0 | 5.3 | ||||||
| Guinea pig | PC-I | 8 | −15.2 | 3.9 | −16.0 | −19.3 | −7.4 | 3.5 | 1.5 | 3.1 | 1.8 | 6.0 |
| Guinea pig | PC-II | 8 | −15.1 | 2.8 | −15.6 | −19.5 | −9.8 | 5.1 | 1.3 | 5.4 | 3.7 | 7.7 |
| ave. | −15.2 | 3.2 | −15.6 | 4.3 | 1.6 | 4.2 | ||||||
| Camelids | PC-I | 5 | −19.6 | 0.9 | −20.0 | −20.2 | −18.0 | 5.0 | 0.2 | 4.9 | 4.8 | 5.4 |
| Camelids | PC-II | 19 | −13.9 | 3.2 | −13.6 | −19.3 | −8.9 | 7.4 | 1.4 | 7.3 | 4.9 | 10.1 |
| ave. | −15.1 | 3.7 | −15.6 | 6.9 | 1.6 | 7.0 | ||||||
| Dog | 1 | −18.4 | — | — | — | — | 7.3 | — | — | — | — | |
| Human (Pacopampa site) | ||||||||||||
| PC-I phase | 9 | −19.3 | 0.4 | −19.4 | −19.8 | −18.9 | 8.3 | 1.0 | 8.3 | 6.6 | 9.9 | |
| PC-II phase (adults and unborn children) | 37 | −17.3 | 1.1 | −17.3 | −19.5 | −15.1 | 7.7 | 0.8 | 7.4 | 6.6 | 9.7 | |
| Cajamarca phase | 1 | −15.6 | — | — | — | — | 9.0 | — | — | — | — | |
| Infant, subadult | 4 | −16.3 | 1.5 | −17.0 | −17.2 | −14.0 | 9.2 | 0.7 | 9.3 | 8.2 | 10.0 | |
| Male | 11 | −16.8 | 1.1 | −16.7 | −19.2 | −15.1 | 7.5 | 0.5 | 7.4 | 6.6 | 8.3 | |
| Female | 18 | −17.2 | 1.0 | −17.3 | −19.1 | −15.4 | 7.4 | 0.6 | 7.3 | 6.9 | 9.3 | |
| N | δ13Cen (‰) | |||||||||||
| Mean | SD | Median | Min. | Max. | ||||||||
| Human (Pacopampa site) | ||||||||||||
| PC-II phase | 8 | −11.0 | 1.3 | −11.2 | −12.4 | −8.8 | ||||||
As mentioned above, the isotope data for our camelid samples showed that they underwent dramatic dietary changes between the Middle Formative Period and the Late Formative Period. This result supports the findings of our previous study of changes in camelid husbandry in the region (Takigami et al., 2020). The δ13Ccol values for camelids were 19.6 ± 0.9‰ and −13.9 ± 3.2‰ in PC-I and PC-II, respectively, and the δ15N values were 5.0 ± 0.2‰ and 7.4 ± 1.4‰, respectively. Both isotope ratios showed significant differences between the two phases (Mann–Whitney U test: P = 1.88 × 10−4 for δ13Ccol and P = 3.29 × 10−4 for δ15N). We also observed a negative correlation between the δ13Ccol and δ15N values in the PC-II phase (Spearman’s rank correlation coefficient: r = −0.829 and P = 1.1 × 10−5). In short, the data indicate that PC-II phase camelids consumed C4 plants. We think that this dietary shift from C3 plants to a mixed diet of C3 and C4 plants indicates changes in their pasture area or the means by which they were fed. Finally, we found δ13Ccol values of −18.4‰ and δ15N values of 7.3‰ for the dog sample. The fact that the δ15N value was slightly higher in this sample than in the other animal samples probably reflects the dog’s higher level of meat consumption.
Human bone collagen and tooth enamelMost of the collagen samples were preserved in good condition. However, as we mentioned above, four samples (07PC-B-H34, 07PC-B-H58, 07PC-B-H59, and 11PC-B2-Ent 518-H1-Individual 2) possessed both poorly preserved collagen and C:N ratios outside the acceptable range (Table S2), and therefore we excluded these samples from further analysis.
Figure 4 shows how the δ13Ccol values of our PC-I and PC-II samples differed. PC-I individuals showed low δ13Ccol values ranging from −19.8‰ to −18.9‰, with an average value and one standard deviation of −19.3 ± 0.4‰ (Table 1). Furthermore, their δ15N values ranged from 6.6‰ to 9.9‰, with a mean of 8.3 ± 1.0‰. By contrast, the δ13Ccol values of PC-II individuals showed more variance: they ranged from −19.5‰ to −14.0‰, with a mean value and one standard deviation of −17.3 ± 1.1‰. The difference in the δ13Ccol values between the PC-I and the PC-II phases was significant, but the difference between their δ15N values was not (Mann–Whitney U test: P = 6.52 × 10−7 for δ13Ccol and P = 0.092 for δ15N). The elevated δ13Ccol values in our samples suggests that PC-II individuals ate more C4 plants and/or terrestrial animals that consumed C4 plants than their PC-I counter-parts.
Carbon and nitrogen isotope ratios of human bones from the Pacopampa site. Ellipses drawn with gray lines show isotope values of food resources in the Andean region (see Figure 2).
Furthermore, these PC-II individuals’ δ15N values ranged from 6.6‰ to 10.0‰, with an average of 7.7 ± 0.8‰. These low δ15N values probably indicate limited consumption of marine resources. We found no significant differences in diet according to gender (Mann–Whitney U test: P = 0.256 for δ13Ccol and p = 0.276 for δ15N). Furthermore, we found that infant and sub-adult samples contained higher δ15N values because of breastfeeding, which causes an increase in heavy nitrogen isotopes, and these individuals exhibited higher trophic levels than their mothers (Mann–Whitney U test: P = 0.004 for δ15N). However, the carbon isotope ratios of infants and sub-adults were not significantly different from those of adults (P = 0.188 for δ13Ccol); therefore, we can assume that these individuals did not use special weaning foods made from C4 plants.
We estimated the contribution of C4 resources using the average values of carbon isotope ratios of food (−24.5‰ for C3 plants and −10.1‰ for C4 plants) which were used to draw a Mahalanobis ellipse. The contribution was 8 ± 3% during PC-I and 23 ± 8% during PC-II, and the highest contribution in PC-II was 38% for the 06PC-C-H25. Although the C3 resources still contributed more than half of the food, it suggests that the C4 resources had been dramatically incorporated into the diet. However, this assumption of end members remains inadequate because it does not take into account the contribution of animal meat. The isotope values of animal meat are different between PC-I and PC-II, and there were also no animals fed C4 plants in PC-I. Therefore, we selected C3 and C4 plants as end members, which were found in both periods and show a small variation in isotope values over time, to estimate the contribution from C4 resources. This estimate only includes direct consumption of C4 plants, and it should be noted that indirect consumption of C4 plants was not considered.
The δ13Cen values ranged from −12.4‰ to −8.8‰, with an average of −11.0 ± 1.3‰ (Table 1). Statistical difference of gender was not significant (Mann–Whitney U test: P = 0.057 for δ13Cen).
The Early Cajamarca phase individuals contained slightly higher δ13Ccol values, which suggests an increase in C4 resource exploitation compared with previous periods. However, as we could obtain collagen in good preservation from only this one individual, this is not a conclusive finding.
The higher δ13Ccol and δ13Cen values found in our PC-II samples indicate that C4 resource consumption increased at the Pacopama site during the Late Formative Period. These C4 resources probably included both C4 plants (carbohydrates) and terrestrial animals (protein). Notably, wild C4 plants native to the Andean region are unsuitable for human consumption—so our PC-II individuals must have consumed domesticated C4 plants. Previous studies have determined the species of C4 plants that were cultivated in the region using starch granule analysis of ceramics and human dental calculus, suggesting the existence of that maize starch granules in PC-I and PC-II (Seki, 2017: 440, analyzed by Vázquez and Tham). Other non-maize starch granules have been found here too, including manioc, frijol, potato, and arracacha. We found no amaranth in our residual starch analyses, and therefore conclude that it is highly likely that the C4 plant that was heavily consumed at Pacopampa was maize.
Our isotope analysis of animal remains indicates that guinea pigs and camelids were consumed as C4 proteins at the site. Furthermore, only domesticated animals showed signs of C4 plant consumption—the δ13Ccol values in our deer remains were low, indicating that wild animals consumed mainly C3 plants. Additionally, because there are hardly any wild C4 plants in the Peruvian highlands (Tieszen and Chapman, 1992; Cadwallader et al., 2012; Szpak et al., 2013), we can assume that guinea pigs and camelids were probably raised by Pacopampa’s inhabitants and fed cultivated C4 plants. Considering the proportion of camelids in the assemblage of animal remains in PC-II, we can assume that camelids were a larger part of the human inhabitants’ diets than guinea pigs (Uzawa et al., 2021). A previous ethnological study reported finding pasturing patterns of llama only in maize fields and not in amaranth fields (McCorkle, 1987). Additionally, it is also unlikely that amaranth was cultivated only for camelid feed, even though humans did not consume it. Therefore, it is appropriate to assume that the cultivated C4 plant consumed by camelids was maize rather than amaranth. These considerations lead us to conclude that the significant C4 resources in Pacopampa were maize and camelids fed with maize.
Our data also indicate that maize may have been consumed during the PC-I phase. In contrast to the isotope ratios of human bone collagen—which suggested C4 resource consumption in PC-II—our guinea pig samples indicated that they consumed C4 plants during PC-I. From this finding, we presume that maize was cultivated to feed both humans and small domesticated animals during PC-I. Although we lack human tooth samples for PC-I—and therefore cannot reliably calculate humans’ C4 energy intake in this phase—it is plausible that maize has been cultivated in Pacopampa since the Middle Formative Period at the latest.
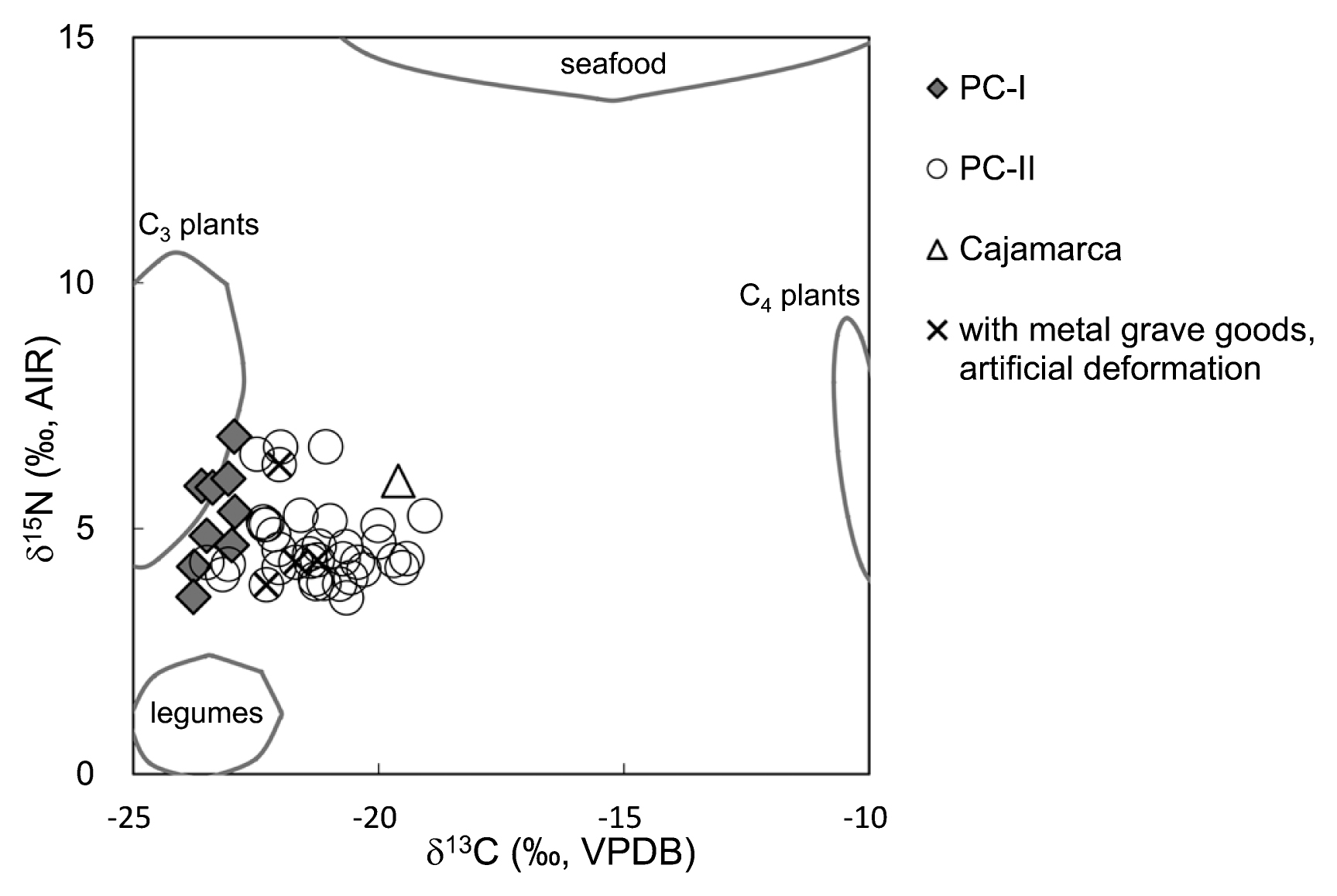
Dietary variation related to social differencesData derived from our collagen and enamel samples of high social class individuals suggested a trend toward lower carbon isotope ratios, but we were unable to detect any significant difference. There were several burials containing metal grave goods (09PC-C-Ent 09-2-H2, 12PC-B2-Ent 532-H1, and 12PC-B2-Ent 530)—which probably indicates that these individuals were of a high social class; the statistical variation in isotope ratios related to social differences was insignificant (Mann–Whitney U test: P = 0.183 for δ13Ccol and P = 0.773 for δ15N; Figure 4). These individuals also had artificial cranial deformations, which might indicate that their social status was determined in their childhood. Although we should consider the possibility that these deformations could signify the social group or groups that the individual originally belonged to, the diet of these individuals (09PC-C-Ent 09-2-H2, 12PC-B2-Ent 527-H1, and 12PC-B2- Ent 532-H1) did not vary significantly from that of the other individuals we collected (Mann–Whitney U test: P = 0.579 for δ13Ccol and P = 0.616 for δ15N).
Furthermore, our δ13Cen data also did not indicate the presence of any dietary variations that we could associate with differences in social status. The δ13Ccol values strongly reflect δ13C protein values. Although maize also contains a small amount of protein, δ13C derived from maize is hidden when animal protein is consumed in significant amounts. We assume that it would be difficult to detect maize ingestion, such as maize grain and chicha, only by analysis of δ13Ccol, owing to the consumption of wild animals that mainly ingested C3 plants (e.g. deer). Additionally, because two individuals buried with metal grave goods (12PC-B2-Ent 532- H1 and 12PC-B2-Ent 530-H1) and one individual exhibiting cranial deformation (12PC-B2-Ent 527-H1) exhibited signs of low C4 resource intake (Figure 5), we suggest that maize did not have tremendous symbolic and social significance during the Late Formative Period.
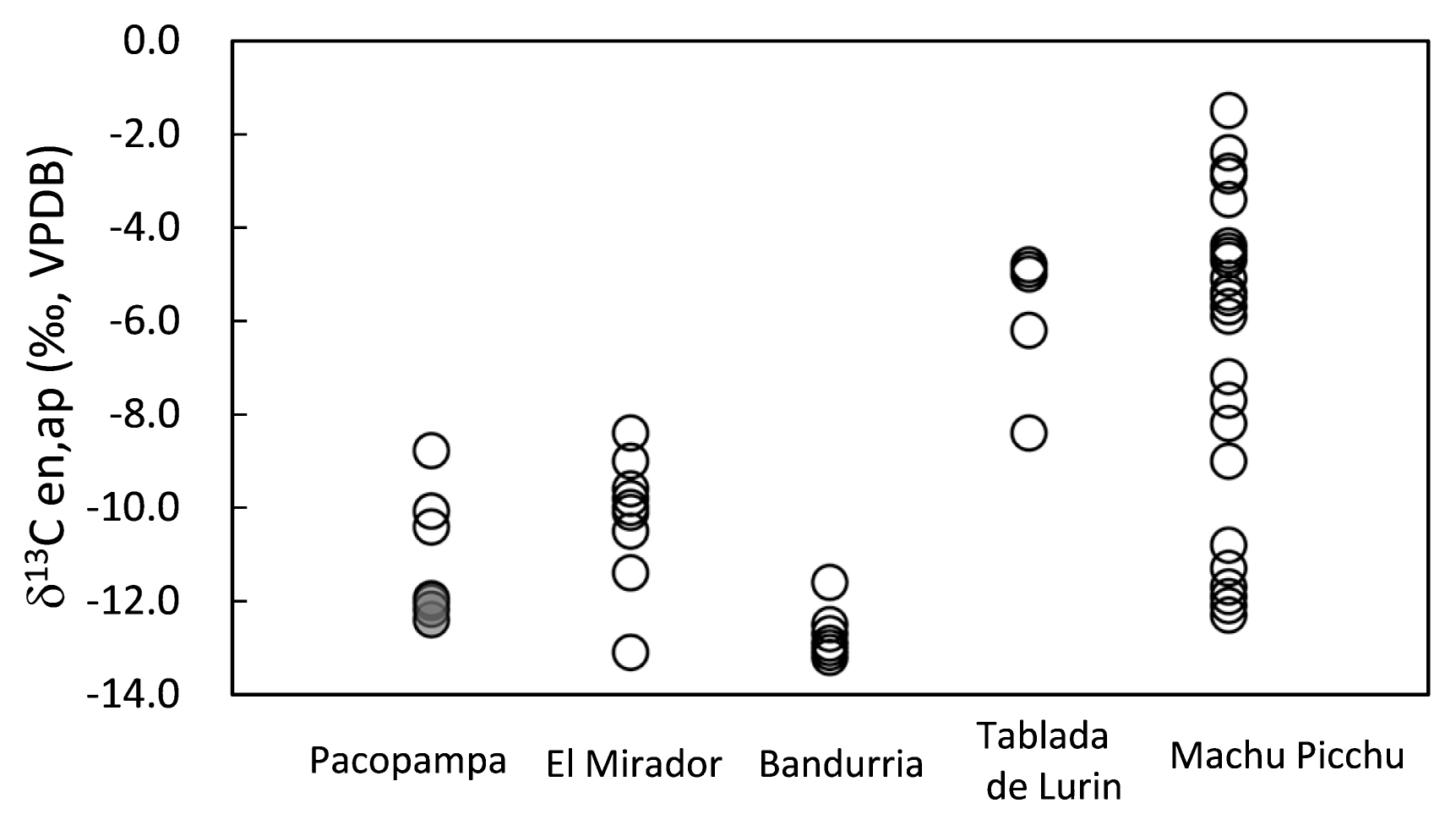
Comparison of the δ13Cen,ap between sites. Three gray marks in Pacopampa indicate high class individuals with metal grave goods or artificial cranial deformation. Isotope data of El Mirador and Tablada de Lurin were cited from Tykot et al. (2006) and Machu Picchu from Turner et al. (2010).
Various studies have revealed that there was a relationship between C4 resource consumption and social status in ancient Andean society (Hastorf and Johannessen, 1993; Hastorf, 2001; Berryman, 2010: 294–300; Larsen, 2015: 312–313). Furthermore, chroniclers noted the importance of maize in redistribution, reciprocity, and ceremonial feasting in the central Andes during the Inca Period. These chroniclers described both the intensive use of chicha and the special status of maize; for instance, Guaman Poma de Ayala wrote that people who were brave consumed both maize and chicha, while people of little strength and courage ate chuñu (dried potato; Guaman Poma de Ayala, 2009 [1615]: 268; Murra, 1973: 397). Some isotope studies have reported dietary variations related to social status. For example, data obtained from child mummies from the Inca period showed that C4 resource consumption increased as sacrificial rituals became more frequent (Wilson et al., 2007). Although these cases are from the Inca Period, when maize agriculture was widespread, we should consider whether maize played such a role during the Formative Period. An isotopic dietary analysis according to social status of high/low-class people at the La Florida site in Ecuador (dated to AD 100–450) showed that high-status individuals who had been buried with various grave goods also ingested more C4 resources than low-status individuals who had been decapitated (Ubelaker et al., 1995).
Unlike these previous studies, our data indicated that high-status individuals consumed slightly fewer C4 resources than their lower-status counterparts. However, this trend was not statistically significant. We might interpret these results by suggesting that maize had not yet been given the political and ceremonial value that it carried during the Inca Period. However, some scholars have suggested that the limited consumption of maize in this period indicates its ritual and symbolic importance, because it was cultivated but was not a primary contributor to the Andean diet (Staller, 2006). In contrast, this study reveals that increases in C4 resource consumption coincided with the emergence of a social hierarchy in Andean society, and that the elites’ consumption of C4 resources was unremarkable; this leads us to conclude that maize likely did not have tremendous importance as symbolic and social food in the Inca Period. Rather, we suggest that the promotion of maize cultivation—and, directly and indirectly, maize consumption—may have started with non-elite groups. Considering the transition of ceramic types in the Cajamarca region (Seki, 1998; Seki and Yoneda, 2005) and the evidence of maize starch granules and phytoliths from artifacts, teeth, and soils collected from ceremonial contexts in the Titicaca Basin (Logan et al., 2012), we should not exclude the possibility that chicha had significant ceremonial and ritual value during the Formative Period. In interpreting these results, however, we should note that all of the individuals analyzed in this study were excavated from the ceremonial terrace of the Pacopampa site. This means that they may all have been elites, whether or not they were buried with grave goods and/or showed artificial cranial deformation. Thus, another interpretation of our findings might be that these people’s diet changed gradually over the approximately 300 years encompassing PC-II.
Dietary difference between sitesOur interpretations must also consider that other studies have detected dietary differences between remains found at Pacopampa and coeval sites. For example, Tykot et al. (2006) reported that some individuals excavated from the El Mirador site—east of the Pacopampa ceremonial architecture site—exhibited low δ13Ccol and high δ13Cap values (δ13Ccol = −19.3 ± 0.4‰, and δ13Cap = −9.0 ± 1.2‰; Figure 2, Figure 5). Thus, the diets of people living at El Mirador were low in C4 proteins and high in C4 carbohydrates/ lipids, implying that they consumed maize. Furthermore, remains from individuals found at Bandurria indicate that they ate a mixed diet of C3 resources and marine resources, and exhibited lower δ13Cap values than remains found at either Pacopampa or El Mirador.
By contrast, remains found at later sites such as Tablada de Lurin and Machu Picchu exhibited higher δ13Cap values, suggesting greater consumption of C4 resources (Figure 5). These difference in the carbon isotope values of collagen and hydroxyapatite suggest changes in C4 resource consumption, including C4 meat, maize grain, and chicha.
We especially focus on the diet of Pacopampa’s neighboring site, El Mirador. Although the relationship between the Pacopampa and El Mirador sites is unclear, the dietary difference between human remains found at these sites may imply that C4 resources were exploited in different ways at each site. This may be a result of social hierarchy or organization at the sites, but it might also be a result of changes in dietary habits over time. Because remains at El Mirador have been dated to 1000–800 BC or 900–600 BC, based on ceramics found at the site, it is possible that the site can be dated to a slightly earlier time than Pacopampa. Furthermore, we cannot reject the possibility of contamination or diagenesis of samples from El Mirador—especially in the case of bone apatite, because bone hydroxyapatite is susceptible to alteration or diagenesis (Koch et al., 1997). Because we noticed isotopic changes in strontium in animal dentine after burial in our previous study (Takigami et al., 2020), we suggest that future researchers expand their investigations around the Pacopampa site in order to better elucidate resource distribution in Andean societies from the Formative Period.
As mentioned above, only a few archaeological sites provide evidence of C4 resource consumption during the Formative Period (Figure 2). Evidence from these sites—Pacopampa, Kuntur Wasi, Pikimachay, and El Mirador—indicates that the initial exploitation and use of C4 resources differed by site and region. However, a pollen analysis in the Cuzco region indicated that maize cultivation probably increased extensively throughout the highland areas of the central Andes during the Late and Final Formative Periods (Chepstow-Lusty, 2011). Our findings lead us to conclude that this trend likely continued and eventually led the symbolic, social, and economic significance of maize to increase over time.
This study performed carbon and nitrogen isotope analyses of human remains from the Pacopampa site in the Peruvian highlands in order to give an overview of dietary changes over time. Its findings suggest that consumption of C4 resources increased during the Late Formative Period, and that C4 resource exploitation was extensively promoted in the central Andes. It also found that dietary variation related to social stratification was insignificant at the Pacopampa site, and suggests that intensive maize cultivation and the symbolic, social, and politic significance of maize were established after the Formative Period.
Maize was used as a carbohydrate resource, livestock feed, and source of chicha. This study highlighted how maize, usually considered a staple as well as a ritual food, indirectly contributed to the human diet because it was used for animal feed during the Formative Period. We infer that maize husks and stems were probably given to domesticated animals after harvests. Furthermore, our data on the diets of guinea pigs suggest that maize may have been cultivated as early as PC-I. In short, although maize agriculture and animal domestication are often discussed separately in the literature, we believe that it is better to discuss them together. In addition, we speculate that that maize harvest remnants were used as feed for camelids, and camelid dung was used as a fertilizer for maize cultivation—thus making maize and maize-fed animals inseparable.
In short, future researchers should discuss maize utilization as both a subsistence strategy and as a means of C4 resource exploitation. We feel that it is important to analyze δ13Ccol, δ13Cap, and δ13Cen together, because δ13Cap and δ13Cen values allow more precise estimates of the contributions that C4 plants make to diets. We think that conducting isotope studies of animal bones simultaneously and/or performing multiple investigations at a single site (and other sites) will address some of this study’s limitations. Because we analyzed only one individual from the Early Cajamarca phase in this study, future studies could undertake a detailed investigation of dietary transitions in the Early Cajamarca phase and refine our interpretations by providing more in-depth investigations of dietary transitions during this period across several sites. By analyzing other functional contexts—such as residential areas—in tandem with ceremonial contexts, we will be able to better elucidate the use of maize in Formative Period societies.
We are deeply grateful to Dr. Megumi Saito-Kano and Nozomi Suzuki at the National Museum of Nature and Science for their support with the carbon isotope ratio analysis of the enamel samples. We thank Ms. Megumi Arata, Ms. Nagisa Nakagawa, and Dr. Wataru Morita for providing invaluable assistance in sample collection. Dr. Atsushi Yamamoto provided useful maps of archaeological sites in Peru. This study was supported by Grants-in-Aid for Scientific Research (KAKENHI nos. 23222003, 16H02729, 16H05639, 19K13398, 20H00050, and 20H01377) and a Grant-in-Aid for JSPS Fellows (no. 12J06868) of the Japan Society for the Promotion of Science (JSPS). Finally, we also would like to thank anonymous referees and editors for useful comments that helped us to improve this paper.