2021 年 18 巻 p. 127-130
2021 年 18 巻 p. 127-130
Photoreceptor proteins utilize light-absorbing cofactor (chromophore) embedded into the protein domain and regulate a variety of biological functions. Understanding how the photoreceptors perceive and transduce the light signal at the atomic level is an important challenge for biophysics. A combination of crystallographic, spectroscopic, and theoretical approaches is crucial to obtain the high-resolution structure of the photoreceptors including protons as well as an electronic structure of the chromophore. To discuss recent state-of-the-art investigations of photoreceptors using these approaches, we held a symposium at the 58th Annual Meeting of the Biophysical Society of Japan in September 2020. In this commentary and perspective, we summarize the discussions on diverse photoreceptor families including cyanobacteriochromes (CBCRs), microbial rhodopsins, and BLUF (blue light using the flavin) proteins.
CBCRs belong phytochrome superfamily of photoreceptors and utilize a linear tetrapyrrole (bilin) chromophore, which is covalently bound to a cysteine residue in the GAF (cGMP-specific phosphodiesterases/adenylyl cyclases/FhlA) domain. Although phytochromes are widely distributed in higher plants, algae, bacteria, and fungi, CBCRs are distributed only among the cyanobacteria [1,2]. CBCRs and phytochromes have two distinct light-absorbing states, whose conversion is triggered by a C15-Z/C15-E photoisomerization at the bilin D-ring. Phytochromes typically photoconvert between a red-absorbing state and a far-red absorbing state, but CBCRs show a marked variation in their absorbing wavelength spanning near-UV to far-red region of the spectrum [1,2]. CBCRs are classified into subfamilies by the C15-Z/C15-E configuration and its corresponding absorbing light colors (e.g. green/red and blue/green subfamilies). Detail molecular mechanisms underlying the spectral diversity of each CBCR subfamily have been elucidated.
RcaE and CcaS belong to the green/red CBCR subfamily that photoconverts between green-absorbing C15-Z state (C15-ZPg) and red-absorbing C15-E state (C15-EPr) [3–5]. This type of CBCRs is conserved in ~15% of all sequenced cyanobacteria genomes to control the light absorption maxima of the photosynthetic antenna complex [6]. Hirose et al. discovered the pH-sensitivity of absorption spectra of this subfamily and demonstrated that the bilin chromophore is deprotonated in C15-ZPg and protonated in C15-EPr [5]. This photocycle was named “protochromic photocycle” since the change in the protonation of the bilin dominantly determines the green or red absorption [5].
Recently, Nagae et al. determined a high-resolution crystal structure of the GAF domain of RcaE in its C15-EPr state (Fig. 1A) [7]. Although phytochromes and CBCRs harbor anti conformation at the bilin D-ring, the RcaE crystal structure shows a unique syn configuration in the C15-EPr state (Fig. 1B). Furthermore, a nuclear magnetic resonance (NMR) analysis demonstrated that the four pyrrole nitrogen atoms are fully protonated in the C15-EPr state, whereas one of the pyrrole nitrogens is indeed deprotonated in the C15-ZPg state.

The crystal structure of RcaE in its C15-EPr state [7] [PDB ID: 7CKV]. A proposed model of the photoconversion of the bilin chromophore is also shown in panel B.
The structure of the deprotonated C15-ZPg state of RcaE has not yet been revealed by crystallography. Osoegawa et al. applied resonance Raman spectroscopy to RcaE in its deprotonated C15-ZPg state [8]. The observed Raman spectra were analyzed with quantum mechanics/molecular mechanics (QM/MM) calculations combined with a molecular dynamics (MD) simulation using a crystal structure of a red/green subfamily AnPixJ2 as a template. The good agreement between experimental and theoretical Raman spectra indicated that the bilin deprotonation occurs at the B-ring nitrogen in the C15-Z,anti structure of RcaE (Fig. 1B).
The bilin deprotonation was also observed for CBCR subfamilies absorbing violet-blue light. These CBCR subfamilies form a second bilin-thiol adduct at the bilin C10 atom between B- and C-rings, which is called “two-Cys photocycle” [9]. Sato et al. explored a photoreaction of a member of blue/orange subfamily and demonstrated a formation of the deprotonated bilin as an intermediate state [10]. Further structural and spectroscopic studies will reveal the generality of the bilin deprotonation in the two-Cys photocycle. One of the challenges of the spectral analysis in CBCRs is the assignment of the complex signals of the bilin chromophore. Kamo et al. established a rapid and efficient method for the bilin-specific 13C and/or 15N labeling and applied the in vitro reconstitution of RcaE [11]. This method will contribute to the spectral assignments in resonance Raman, Fourier-transform infrared (FTIR), and NMR analyses in other diverse CBCR subfamilies.
One of the other important topics in the symposium was a microbial rhodopsin, which is composed of seven trans- membrane helices and a retinal chromophore bound to a specific lysine residue via Schiff base linkage. Upon illumination, retinal isomerizes from an all-trans to a 13-cis configuration, leading to a cyclic reaction with several intermediate states. The most abundant function of microbial rhodopsins is a light-driven ion pump, e.g., H+ and Na+ pumpings for Gloeobacter rhodopsin (GR) and a sodium-pump rhodopsin (NaR), respectively. For these membrane transporters, substrate (ion) uptake and release reactions are crucial events for their function. Methods for measurement of proton transfer have been established spectroscopically or electrochemically [12]. By contrast, in NaRs, it is difficult to identify their relevant intermediates because of a difficulty to detect a substrate movement. Recently, Kikukawa and co-workers achieved this difficult task by developing a Na+-selective membrane that consists of polyvinyl chloride and a Na+ ionophore [13]. The application of this novel technique to NaR allowed them to observe a light-induced change in membrane potential, which reflects the transient Na+ uptake and release reactions (Fig. 2A). Furthermore, they measured flash-induced absorbance change in NaR to detect the formation and decay of intermediate states. The good agreement between the two kinetic events indicated that Na+ is captured and released by NaR during the formation and decay of the O intermediate.
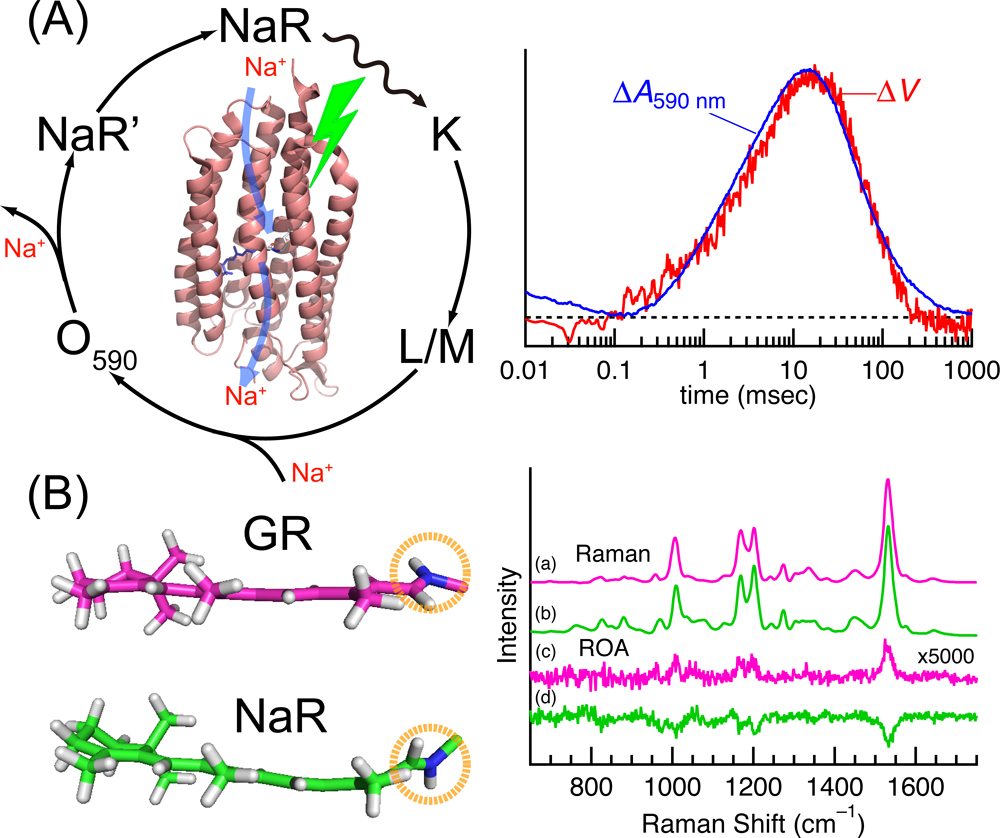
Two biophysical approaches for microbial rhodopsins. (A) A photocycle of NaR and time courses of O accumulations and Na+ concentration changes [13]. (B) Structures of the retinal chromophore in GR and NaR. The orientation of the Schiff base NH moiety (marked in orange circles) determines the sign of the ROA spectra [14].
Recently, low-temperature Raman spectroscopy has been applied to several microbial rhodopsins to reveal the active site structures in an early photointermediate [15–17]. This method allows us to examine the primary structural change of the retinal chromophore upon photoexcitation. For example, the application of this method to NaR revealed that the light illumination produces the distorted 13-cis retinal chromophore in the presence of Na+, whereas the structural distortion of the chromophore is significantly relaxed without Na+ [17]. The structural difference of the chromophore in the presence or absence of Na+ was ascribed to the binding of Na+ to the protein. This finding provided new insight of Na+ binding for active Na+ transport in NaR.
Raman optical activity (ROA) is an emerging spectroscopic technique that can be applicable to photoreceptor proteins including microbial rhodopsins [14,18–20]. It provides chiral sensitivity and measures the right and left circularly polarized components in Raman scattered lights [21]. Recent progress showed that this method is promising to detect the distortion of a chromophore within a protein environment [22–24]. The crystal structure of GR demonstrates that its protonated Schiff base NH moiety forms a hydrogen bond with a water molecule [25]. On the other hand, the Schiff base NH group forms a hydrogen bond with an aspartate near the chromophore. This ionic hydrogen bond makes the retinal NH moiety to point toward the aspartate, leading to an oppositely out-of-plane distorted chromophore in NaR compared to that of GR (Fig. 2B). Matsuo et al. showed that the ROA spectra of GR and NaR are opposite in signs, demonstrating that the twist direction of the retinal chromophore determines the sign of the ROA spectrum [14]. Because the environments of the Schiff base moiety is important for functions, the ROA method provides a powerful approach to detecting functionally important conformational details in a protein active site.
Another important class of photoreceptor proteins uses a flavin such as flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) as a chromophore. Three types of photoreactions are known for flavin-binding photoreceptor proteins. The BLUF domain exhibits a unique photoreaction, in which the chromophore does not change its chemical structure between unphotolyzed and intermediate states. A possible mechanism for the photoreaction of the BLUF domain involves a keto-enol tautomerism of a conserved Gln residue. In order to explore the unique mechanism, Iwata et al. applied light-induced difference FTIR spectroscopy to the BLUF domain of AppA [26] (see Fig. 3). They used isotopically labeled samples including 15N-Gln, and the effects of the isotope labelings on the difference FTIR spectra were examined. The observed FTIR data were interpreted from QM/MM calculations. These combined efforts demonstrated that the photoreaction of AppA-BLUF involves the keto-enol tautomerization and rotation of the Gln side chain.
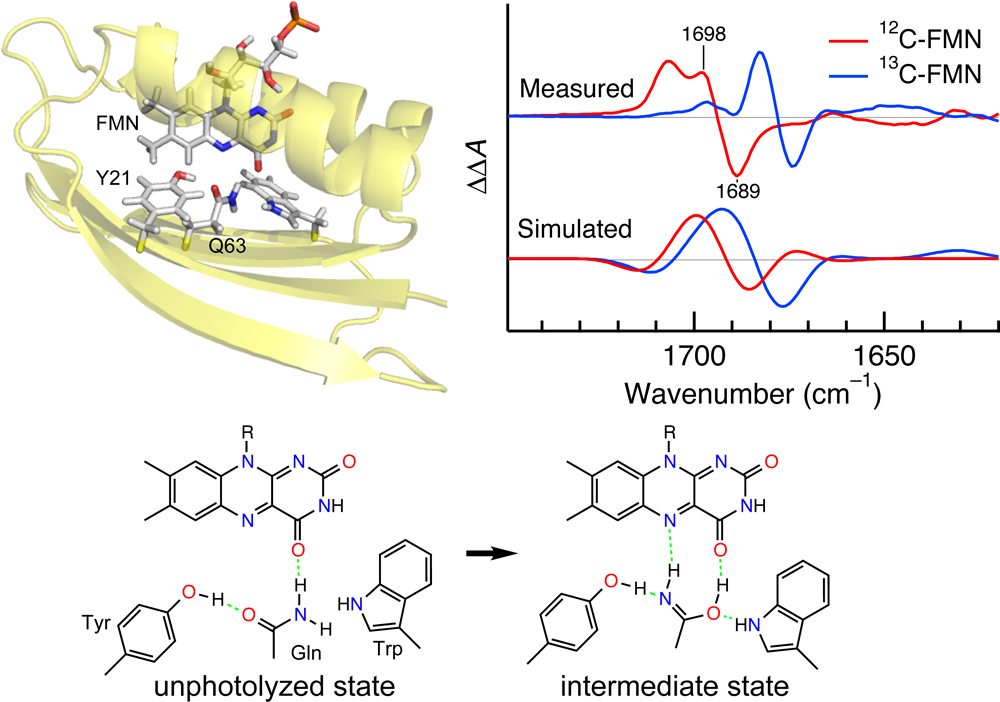
Observed and simulated difference FTIR spectra for the BLUF domain of AppA. The upper left panel shows a QM/MM structure of the AppA-BLUF domain in its dark state. The signals from nitrogen-related vibrations of Gln were extracted in the presence of 12C-FMN and 13C-FMN. The lower panel shows a proposed photoconversion model [26].
The light-triggered nature of photoreceptors allows studies on the protein structures and functional protein dynamics using a wide range of biophysical methods. The systems discussed here included bilin-binding proteins such as CBCR, microbial rhodopsins, and flavin-binding BLUF proteins. We believe that future studies using the state-of-the-art techniques discussed in the symposium for these systems will derive general principles for the molecular mechanisms that realize protein functions.