2023 年 20 巻 1 号 論文ID: e200003
2023 年 20 巻 1 号 論文ID: e200003
Some evidence suggests that oxytocin, which is a neuropeptide conventionally thought to be synthesized in the hypothalamus and released by the posterior pituitary, is generated in peripheral keratinocytes, but the details are lacking and the mRNA analysis is further required. Oxytocin and neurophysin I are generated together as cleavage products after splitting the precursor molecule, preprooxyphysin. To confirm that oxytocin and neurophysin I are also generated in the peripheral keratinocytes, it must first be clarified that these molecules contained in peripheral keratinocytes did not originate in the posterior pituitary gland and then the expression of oxytocin and neurophysin I mRNAs must be established in keratinocytes. Therefore, we attempted to quantify preprooxyphysin mRNA in keratinocytes using various primers. Using real-time PCR, we observed that the mRNAs of both oxytocin and neurophysin I were located in keratinocytes. However, the mRNA amounts of oxytocin, neurophysin I, and preprooxyphysin were too small to confirm their co-existence in keratinocytes. Thus, we had to further determine whether the PCR-amplified sequence was identical to preprooxyphysin. The PCR products analyzed by DNA sequencing were identical to preprooxyphysin, finally determining the co-existence of both oxytocin and neurophysin I mRNAs in keratinocytes. In addition, the immunocytochemical experiments showed that oxytocin and neurophysin I proteins were located in keratinocytes. These results of the present study provided further support indicating that oxytocin and neurophysin I are generated in peripheral keratinocytes.
Oxytocin is produced in the brain, and enhances social bonding (e.g., emotional recognition and empathy) and improves symptoms in autistic patients. Oxytocin is also involved in pain relief mechanisms in the central and peripheral nervous systems. This leads to the question of whether peripheral oxytocin also derives from the brain or is generated in the periphery. Although previous reports revealed the presence of oxytocin protein in peripheral keratinocytes, the present study carefully evaluated the presence at the mRNA level, thereby establishing that oxytocin is generated in peripheral keratinocytes.
Oxytocin (OXT), a 9 amino-acid neuropeptide, is synthesized in neurosecretory cells in the paraventricular and supraoptic nuclei of the hypothalamus, and secreted from the posterior pituitary [1–3]. OXT is generated together with neurophysin (NP) I as these are both cleavage products generated by splitting the precursor molecule, preprooxyphysin [4]. OXT stimulates uterine contractions during labor and mammary gland muscle fiber contractions for milk secretion. This is the historical understanding of OXT, as described in most neuroendocrinology textbooks [4]. Recently, OXT is also secreted upon establishing positive social bonding (i.e., interpersonal relationships) [5]. The secretion of OXT decreases the desire to fight or to flee and reduces fear; thus, it is often referred to as the “hormone of happiness” [6]. Although the entire hypothalamic-pituitary-adrenal axis (HPA axis) is involved in these functions [7], it is considered that the synthesis and secretion of this OXT occur in the brain and not the periphery.
OXT is also reported to be involved in pain relief [8]. Our research group is also one of them [9–12], and has already confirmed the presence of OXT and OXT receptors in the dorsal root ganglion (DRG) connecting the central nervous system and the periphery, indicating that mechanoreception and nociception may induce the autocrine/paracrine function of OXT in the DRG and thus contribute to pain relief [13,14]. That is, OXT may be produced in DRG neurons when the skin is gently dabbed, thereby contributing to pain relief. Our research group also confirmed that intracellular Ca2+ propagates from peripheral keratinocytes to DRG neurons via gap junctions [15] in addition to the ATP-mediated pathway, which is generally thought to be involved in the transmission of information in keratinocytes and DRG neurons [16]. In other words, the information of the physical stimulus is transmitted from keratinocytes to DRG neurons via multiple pathways. Therefore, if OXT is synthesized in peripheral keratinocytes, it is reasonable to assume that the OXT can be coherently used for pain relief mechanisms in keratinocytes, DRG neurons connecting the periphery and the central nervous system, and the brain.
S. Denda and colleagues reported that OXT is expressed in keratinocytes [17]. Furthermore, Deing and colleagues reported that OXT and OXT receptors are expressed not only in keratinocytes but also in fibroblasts [18]. The OXT in fibroblasts has been investigated for its role in suppressing senescence [19]. Whereas these studies revealed the presence of OXT mRNA and protein in keratinocytes, few previous studies examined the detailed synthetic machinery needed for oxytocin synthesis in keratinocytes. The mRNA amounts of OXT and NP I are too small, as described later, to determine their co-existence in keratinocytes. This prompted us to re-examine the evidence for oxytocin synthesis in peripheral keratinocytes. To confirm that OXT and NP I are co-expressed in keratinocytes, it is important to investigate the presence of the mRNA, not just the protein. Therefore, we first attempted to quantify preprooxyphysin mRNA with real-time PCR using various primers. We must repeatedly note that OXT is generated together with NP I, because both of these are cleavage products after splitting of the compound molecule, preprooxyphysin. We next evaluated whether the amplified mRNA sequence was identical to that of preprooxyphysin. Using real-time PCR, we quantitatively investigated OXT, NP I, and preprooxyphysin mRNAs in detail. Evidence at the protein level was also confirmed by immunocytochemistry. Our results confirmed that OXT is synthesized in keratinocytes.
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at Waseda University (2021-A004). Wistar rats (postnatal day 5) were anesthetized with sevoflurane (Fujifilm Wako Pure Chemical). The head and limbs were amputated. The pituitary gland was collected. The skin of the body was cut along the midline of the back, peeled off, and placed in 0.4% (w/v) dispase (Godoshusei) in HBSS and shaken for 12–18 h at 4°C. The skin was washed with 2 mL PBS, placed on a 96-mm dish with the fur facing down, and 500 μL TrypLE Select (Gibco) was added. Only the thin skin was transferred to a new dish. The thin skin was placed with the fur facing up and 500 μL TrypLE was added. The thin skin was shaken for 20 min at room temperature, then 2 mL CnT-Prime, Epithelial Culture Medium (CnT-PR; CELLnTEC) was added, and the preparation was shaken further. The suspension was collected in a 50-mL tube through a 100-μm filter (EASYstrainer). This collection procedure was conducted 3 times. The collected suspension was centrifuged at 200×g for 5 min at room temperature. The supernatant was removed and 1 mL CnT-PR was added to the suspension. Then, 1×106 cells were seeded on 60-mm culture dishes (Violamo, AsOne).
RNA Extraction and Real-Time PCRKeratinocytes were washed twice with PBS. Total RNA was extracted from keratinocytes with a ReliaPrep RNA Miniprep System (Promega). We also extracted total RNA from the pituitary gland using Isogen II (Nippon Gene) following the manufacturer’s protocol. The total RNAs were reverse-transcribed by ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo). Real-time PCR was performed with StepOnePlus (Applied Biosystems) using THUNDERBIRD Next SYBR qPCR Mix (Toyobo). We defined RQ values as 2–ΔCt×1000. The primers used are listed in Table 1. The reference genes used were β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). As shown in a previous study, β-actin and GAPDH expressions were stable [13].
| Primer | Sequence (5'–3') | Accession number |
|---|---|---|
| OXT_F | TCTCGGACTGAACACCAACG | NM_012996.3 |
| OXT_R | AGTTCTGGATGTAGCAGGCG | |
| NP_F | CTGCGCTAGACCTGGATATG | NM_012996.3 |
| NP_R | GTAGTTCTCCTCCTGGCAGC | |
| OXT-NP_F | TCTCGGACTGAACACCAACG | NM_012996.3 |
| OXT-NP_R | GTAGTTCTCCTCCTGGCAGC | |
| Sequence_F | TCTCGGACTGAACACCAACG | NM_012996.3 |
| Sequence_R | GTAGTTCTCCTCCTGGCAGC | |
| β-actin_F | TGTCACCAACTGGGAC | NM_007393.5 |
| β-actin_R | GGGGTGTTGAAGGTCT | |
| GAPDH_F | TATGACTCCACTCACGGCAAAT | NM_001289726 |
| GAPDH_R | GGGTCTCGCTCCTGGAAGAT |
OXT: oxytocin, NP: neurophysin I, OXT-NP: see Figure 1, F: forward, R: reverse.

Primer design of OXT and NP I. Three pairs of primers were designed for real-time PCR. (a) OXT-NP: This primer was designed to cover both the OXT and NP I coding sequences, which correspond to preprooxyphysin. (b) OXT: This primer was designed for OXT and its upstream sequence. (c) NP: This primer was designed for NP I and its downstream sequence. Preprooxyphysin mRNA is 534 bp long (NM_012996.3, NCBI), in which the 94th–120th portion encodes the mat peptide of OXT and the 130th–156th encodes that of NP I. The PCR target for OXT was 95 bp in the 15th–109th portion, that for NP was 146 bp in the 131st–276th, and that for OXT-NP was 262 bp in the 15th–276th.
cDNA prepared from keratinocytes was amplified by KOD FX (Toyobo). The amplified product was again amplified by Ex Taq (Toyobo). The primers used for both amplifications were Sequence F and Sequence R, shown in Table 1. The final products were consigned to Eurofins sequence analysis (https://eurofinsgenomics.jp/jp/service/dnasequence/order-start.aspx).
ImmunocytochemistryThe supernatant was removed from the keratinocyte culture, as mentioned above, and the cells were washed twice with 1 mL PBS. Keratinocytes were fixed with 1 mL of 4% paraformaldehyde in PBS. The supernatant was removed, and the cells were washed 3 times with 1 mL PBS containing 0.05% Tween-20 (PBS-T). Then, 1 mL 0.1% Triton X was added to the cells and incubated with them for 10 min at room temperature. The supernatant was removed and the cells were washed 3 times with 1 mL PBS; 500 μL of 3% bovine serum albumin (BSA) in PBS-T was added, and the cells were incubated further for 30 min at room temperature. The supernatant was then carefully removed.
For keratin staining, anti-cytokeratin 14 antibody (1:1000; LL002, abcam), as the primary antibody, was used in 3% BSA in PBS-T. The reaction was carried out overnight in the dark at 4°C. After removing the supernatant, goat anti-mouse IgG H&L (Alexa Fluor 568; 1:1000; ab175473, abcam), as the secondary antibody, and DAPI (1:1000; MilliporeSigma) in 3% BSA in PBS-T were simultaneously added, and the cells were incubated in the dark for 1 h at room temperature. For OXT and NP I staining, anti-oxytocin antibody (1:1000; EPR20973, abcam), as the primary antibody, and anti-NP I antibody (1:1000; clone PS 38, MilliporeSigma), as the primary antibody, were simultaneously used in 3% BSA in PBS-T. The reaction was carried out overnight in the dark at 4°C. After removing the supernatant, goat anti-rabbit IgG Superclonal Recombinant Secondary Antibody (heavy chain, Alexa Fluor 488; 1:1000; Invitrogen) as the secondary antibody for OXT, goat anti-mouse IgG H&L (Alexa Fluor 568; 1:1000; ab175473, abcam) as the secondary antibody for NP I, and DAPI (1:1000; MilliporeSigma) in 3% BSA in PBS-T were simultaneously added, and the cells were incubated in the dark for 1 h at room temperature.
For microscopic observation, the supernatant was removed and the cells were washed 3 times with 1 mL PBS-T. Then the cells were sealed in Shandon Immu-Mount (Thermo Fisher Scientific) and observed under a fluorescence microscope (FV1000, Olympus).
Statistical AnalysisThe data are expressed as the mean±SEM.
Real-time PCR was performed to evaluate the mRNA expression levels of preprooxyphysin, OXT, and NP I. For keratinocytes, RQ for OXT=11, RQ for NP=3, and RQ for OXT-NP=2, and for the pituitary gland, RQ for OXT=1861, RQ for NP=2573, and RQ for OXT-NP=3044. Then, these mRNAs were compared between those isolated from keratinocytes and the pituitary gland. The mRNA expression levels of preprooxyphysin, OXT, and NP I were much lower in keratinocytes than in the pituitary gland (Figure 2). Therefore, at this stage, we could not judge whether these 3 mRNAs were generated in the keratinocytes, and further investigation was performed at the mRNA level.

Relative expression levels of preprooxyphysin, OXT, and NP I mRNAs in keratinocytes (relative expression level [RQ]±SEM, n=9 each) and pituitary gland for the control (RQ, n=1 each). We amplified the cDNAs using 3 pairs of primers. (a) OXT-NP I. This molecule corresponds to preprooxyphysin mRNA. (b) OXT. (c) NP I. The reference genes were β-actin and GAPDH. The expression levels are presented as the data of keratinocytes are set as 1 because the mRNA levels of keratinocytes were so low.
Because of the low expression levels of OXT, NP I, and preprooxyphysin mRNAs, the PCR products required careful examination. Thus, the PCR products were sequenced by DNA cloning to confirm their contents. Figure 3 shows that OXT, NP I, and preprooxyphysin mRNAs obtained by PCR were identical to those obtained from the brain (NM_012996.3, NCBI). Even though the expression levels of OXT, NP I, and preprooxyphysin mRNAs were low in keratinocytes, all 3 were present. The simultaneous presence of these 3 mRNAs in keratinocytes indicates that OXT is generated in keratinocytes.
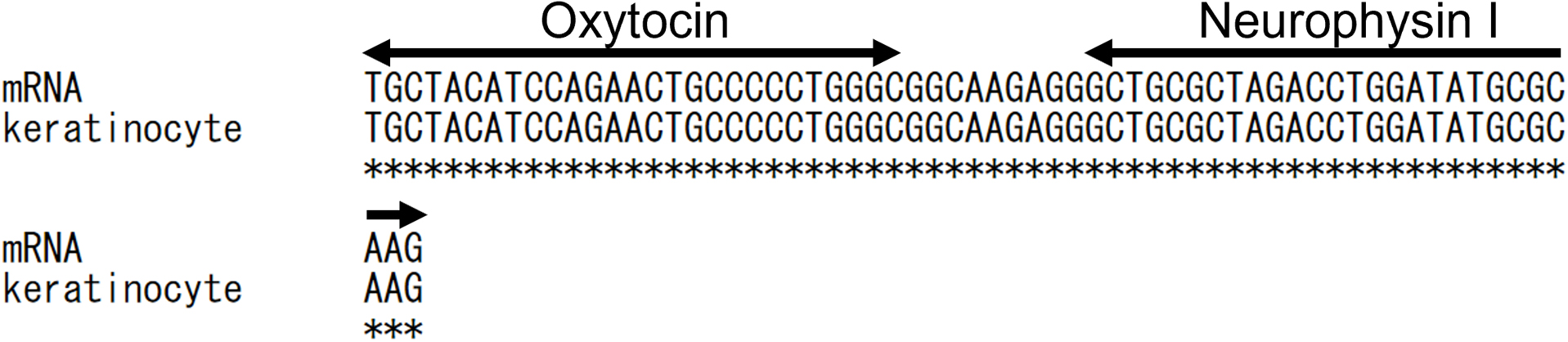
Alignment of OXT and NP I mRNAs derived from keratinocytes. The upper sequence (mRNA) was obtained from a database (NM_012996.3, NCBI), and the lower sequence (keratinocyte) was obtained by us. The OXT and NP I coding sequences are indicated by the double-headed arrows. * indicates the identical base.
The presence of OXT and NP I proteins was confirmed by immunohistochemistry. First, to confirm that the collected cells were keratinocytes, we stained for the presence of keratin, which is specifically contained in keratinocytes. Positive keratin staining confirmed that the collected cells were keratinocytes (Figure 4).

Immunocytochemistry of cells collected from thin skin using a keratinocyte-specific antibody. (a) DAPI staining, (b) Anti-keratin antibody staining. (c) Merge. Scale bars: 30 μm.
Next, the collected cells were evaluated by immunocytochemistry to determine whether OXT and NP I proteins were present. As shown in Figure 5, OXT and NP I proteins were labeled in all the keratinocytes examined.

Immunocytochemistry of cells collected from thin skin using OXT- and NP I-specific antibodies. (a) DAPI staining, (b) Anti-OXT antibody staining, (c) Anti-NP I antibody staining, (d) Merge. Scale bars: 30 μm.
The present results clearly demonstrated the presence of OXT, NP I, and preprooxyphysin at the mRNA level and the presence of OXT and NP I proteins in keratinocytes. The immunocytochemical results showed that there might be plenty of OXT proteins in the keratinocytes, whereas the PCR results showed that there was only a trace amount of OXT mRNA. In other words, this result means that immunocytochemistry has no quantitative property. We do not know how many proteins are present even if the staining conditions are optimized in immunohistochemistry. Thus, it is not possible to determine that OXT is generated in peripheral keratinocytes without a detailed examination at the mRNA level. Although the mRNA amounts in keratinocytes were very small, we were able to rule out the possibility that the molecules are generated only in the brain and coincidentally present in peripheral keratinocytes using the detailed PCR analysis.
OXT is reported to be involved in pain relief. Furthermore, OXT produced in keratinocytes may also be involved in reducing the repetitive behavior of patients with autism and Asperger’s syndrome and the mental state effects [20]. Interestingly, a recent study demonstrated that OXT application does not impair skin wound healing or cell behavior [21].
Whereas previous studies reported that keratinocytes contain OXT, we further demonstrated the presence of both OXT and NP I mRNAs and their proteins in keratinocytes by real-time PCR and immunocytochemistry, respectively. The PCR products were analyzed with DNA sequencing, confirming the co-existence of both OXT and NP I mRNAs in keratinocytes, although the amounts were very small. These findings confirm that the OXT and NP I present in keratinocytes are synthesized in the peripheral keratinocytes, and are not brain-derived.
The authors declare no conflict of interest.
Conceptualization, E.I.; methodology, K.F. and K.I.; investigation, K.F. and K.I.; writing—original draft preparation, K.F., K.O. and E.I.; writing—review and editing, K.I.; project administration, E.I.; funding acquisition, K.O. and E.I. All authors have read and agreed to the published version of the manuscript.
The evidence data generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
This study was supported by a Grant-in-Aid (KAKENHI) for Scientific Research (A) from the Japan Society for the Promotion of Science to K.O. and E.I. (19H00633). The funder had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.