2023 Volume 20 Issue Supplemental Article ID: e201005
2023 Volume 20 Issue Supplemental Article ID: e201005
Microbial rhodopsin is basically composed of seven transmembrane helices and binds an all-trans retinal as the chromophore through a protonated Schiff base linkage with a specific lysin residue on the seventh helix [1,2]. Upon specific wavelength of light activation, isomerization of the retinal chromophore from all-trans to 13-cis configuration initiates the cyclic photoreaction (Figure 1a). Initially, four kinds of microbial rhodopsins were found from an extremely halophilic archaeon, Halobacterium salinarum, and extensive studies were conducted; they are bacteriorhodpsin (BR), halorhodopsin (HR), sensory rhodopsin I (SRI), and sensory rhodopsin II (SRII). BR functions as an outward proton pump [3], while HR functions as inward chloride ion pump. They both are regarded as ion transporting rhodopsins. On the other hand, SRI and SRII each functions as light sensors regulating the positive and negative phototactic behavior of a bacterial cell, respectively. Since 2000, the metagenomic analysis opened new worlds of microbial rhodopsins in the ocean, fresh water, soils, etc. Nowadays, various kinds of functions, including but not limited to light-driven ion pump, light sensor, light-gated channel, light-regulated enzyme, and more are identified in new microbial rhodopsins (Figure 1b) [2,4]. In this brief review, ion-transporting rhodopsins are main focus.
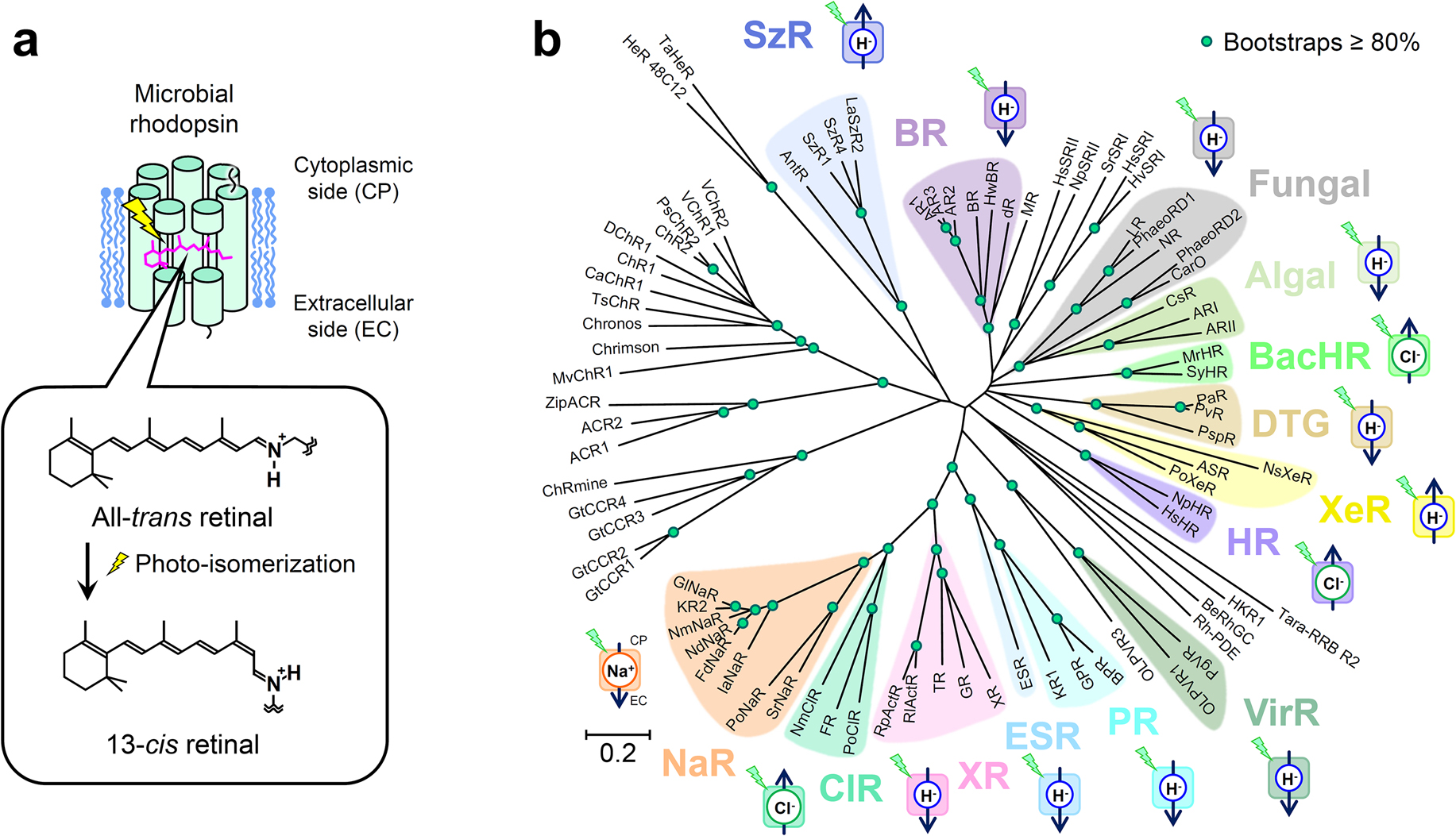
Basic architecture and photochemistry of microbial rhodopsin (a) and a phylogenetic tree (b) including some typical microbial rhodopsins. Ion-transporting rhodopsins are highlighted with schematic figures showing direction of ion transport and a type of transposable ions. This figure was generously provided by Prof. Keiichi Inoue in the University of Tokyo.
An outward proton-pumping rhodopsin generates proton gradient and forms electrochemical potential or proton mobile force (PMF) across the cell membrane, which is utilized by ATP synthase and flagellar motor for biological consumable energy generation. Thus, it is not so surprising that they were found from bacteria living in aquatic environments like salt lake and the ocean. One of the examples is proteorhodopsin (PR); it has been found from many ocean cyanobacteria and mainly contributes on the generation of bioenergy on the earth by harvesting light energy from the sun (~1013 W) in addition to chlorophyll-based photosynthetic systems [2,5]. Another interesting feature was found in xanthorhodopsin (XR), which binds a carotenoid as antenna capturing light energy and transferring the energy to the nearby retinal chromophore [6]. Although biological function remains elusive, a fungal pathogen (Leptosphaeria maculans) causing leaf disease of plant also possesses outward proton-pumping rhodopsin (LR) [7].
For unicellular organism, inward proton pump seems to be useless at first, because it dissipates the proton gradient and hence electrochemical potential or PMF utilized for living. When a single point mutation (D217E) of Anabaena sensory rhodopsin (ASR) produces inward proton-pumping activity [8,9], it was not anticipated that such inward proton pump exists in nature as ASR was considered a light sensor for regulating gene expression. But in 2016, the first inward proton pump in nature was found from a deep-ocean marine bacterium, Parvularcula oceani, and named as xenorhodopsin (XeR) [10]. Then in 2020, another inward proton pump, schizorhodopsin (SzR), was discovered from Asgard archaea, which is the archaeal group closest to eukaryotes [11]. It turns out those light-driven inward proton pump rhodopsins are widely distributed in nature.
It had been widely believed that microbial rhodopsin possesses a single chromophore in monomer until XR, an outward proton pump, was found with a secondary chromophore, salinixanthine, which functions as antenna chromophore capturing blue to green light in the range of wavelength 430 to 530 nm [6]. Upon the light absorption by salinixanthine antenna, the excited energy is transferred to the retinal chromophore for the photo-isomerization and initiates the photocycle of XR. Gloeobacter rhodopsin (GR) [12] and thermophilic rhodopsin (TR) [13] are other microbial rhodopsins located in the same clade. They both also bind two carotenoids, and harvest the light energy in a similar mechanism to XR [14,15]. It should be noted that TR also attracts researchers’ attention for its high thermal stability [16].
In 2013, another breakthrough came in ion transporting rhodopsin: light-driven cation transportation was discovered. Due to electric repulsion between cations and the protonated retinal Schiff base (PRSB) in microbial rhodopsin, it had been believed that cations can not be transported by microbial rhodopsin except of proton. For the first time, Krokinobacter rhodopsin 2 (KR2) was characterized as outward sodium and proton hybrid pump [17]. In the presence of sodium ions, KR2 pumps sodium ion outwardly, while it pumps protons in the absence of sodium ion or the presence of cation species larger than sodium ion, i.e. K+, Rb+, Cs+ and so on. In the natural marine condition, KR2 pumps exclusively sodium ion, because sodium ion (~0.5 M) are more abundant than proton (~10–8 M) [18]. Thus, the clade including KR2 is classified to sodium pumping rhodopsin (NaR).
Halorhodopsins from Halobacterium salinarum (HsHR) and Natronomonas pharaonis (NpHR) were extensively studied as inward chloride ion pumps from traditional microbial rhodopsin. A chloride ion binds at the PRSB as its counter ion instead of either deprotonated Asp or Glu residue, which is well conserved in outward proton pump rhodopsins (D85 in BR). By replacing D85 in BR to Thr or Ser, BR was converted to chloride ion pump [19]. Therefore, the basic machinery of chloride ion pump is preserved in outward proton pump rhodopsin. Recently, the first chloride pumping rhodopsin from bacteria has been identified in marine flavobacterium, Nonlabens marinus S1-08T, and it was classified as a new inward chloride pump and called “ClR” [20]. Interestingly, NmClR has Asn at the corresponding position of D85 in BR instead of Thr. Another group of inward chloride pump was discovered from cyanobacteria and tentatively named as “BacHR”. The first one was identified in Mastigocladopsis repens (MrHR) [21,22] and the other one was in Synechocystis sp. PCC 7509 (SyHR) [23]. It is intriguing that SyHR can pump a divalent anion, SO42– as well [23]. Both MrHR and SyHR loses Asp residue at the corresponding position of D85 in BR and possess Thr as similarly to HsHR and NpHR.
For ion transporting rhodopsins, three amino acid residues on the third transmembrane helix (TM3) are important to exert their functions (Figure 2). In the case of BR, which is a well characterized outward proton pump, D85, T89, and D96 correspond to the three amino acid residues, and was named DTD motif. Most of outward proton pump conserved such DTD or DTE motif with the exception of DTK in ESR and DTG/DTS in newly found groups from bacteria (DTG/DTS rhodopsins) and virus (VirR). The side chains of Lys, Gly, and Ser lack a carboxyl group which serves as a proton donor for the Schiff base in the other outward proton pump rhodopsins. The molecular mechanisms of the proton transfer in the cytoplasmic side were investigated in these exceptional rhodopsins. Chloride pumping rhodopsins lack Asp or Glu at the primary counter ion for the PRSB as those D85S or D85T mutant in BR. Deletion of the negative charge and introduction of hydrophilic group (a hydroxyl group in Thr and Ser) on that position is prerequisite for binding of a chloride ion as substrate for transportation. However, Thr at this position does not always facilitate chloride ion binding as marine bacterial TAT rhodopsin does not bind a chloride ion [24,25]. Inward proton pump, SzR, replaces the carboxyl group with a bulky hydrophobic residue (Phe), and it cannot bind a chloride ion near the PRSB. Interestingly, inward proton pump, XeR, which locates at another clade of the phylogenetic tree, has a conserved Asp residue to serve as the primary counter ion; however it lacks the secondary counter ion at the seventh helix. Therefore, two asp residues serving as the counter ions are required for such outward proton pumping function. Sodium-pump rhodopsins (NaRs) also lack an Asp residue at the former conserved position, but they possess an Asp locates one helical turn above. Thus, NaRs share the NDQ motif. Transient deprotonation of the PRSB and its immediate back reaction may be important for passing through sodium ion in the photocycle.
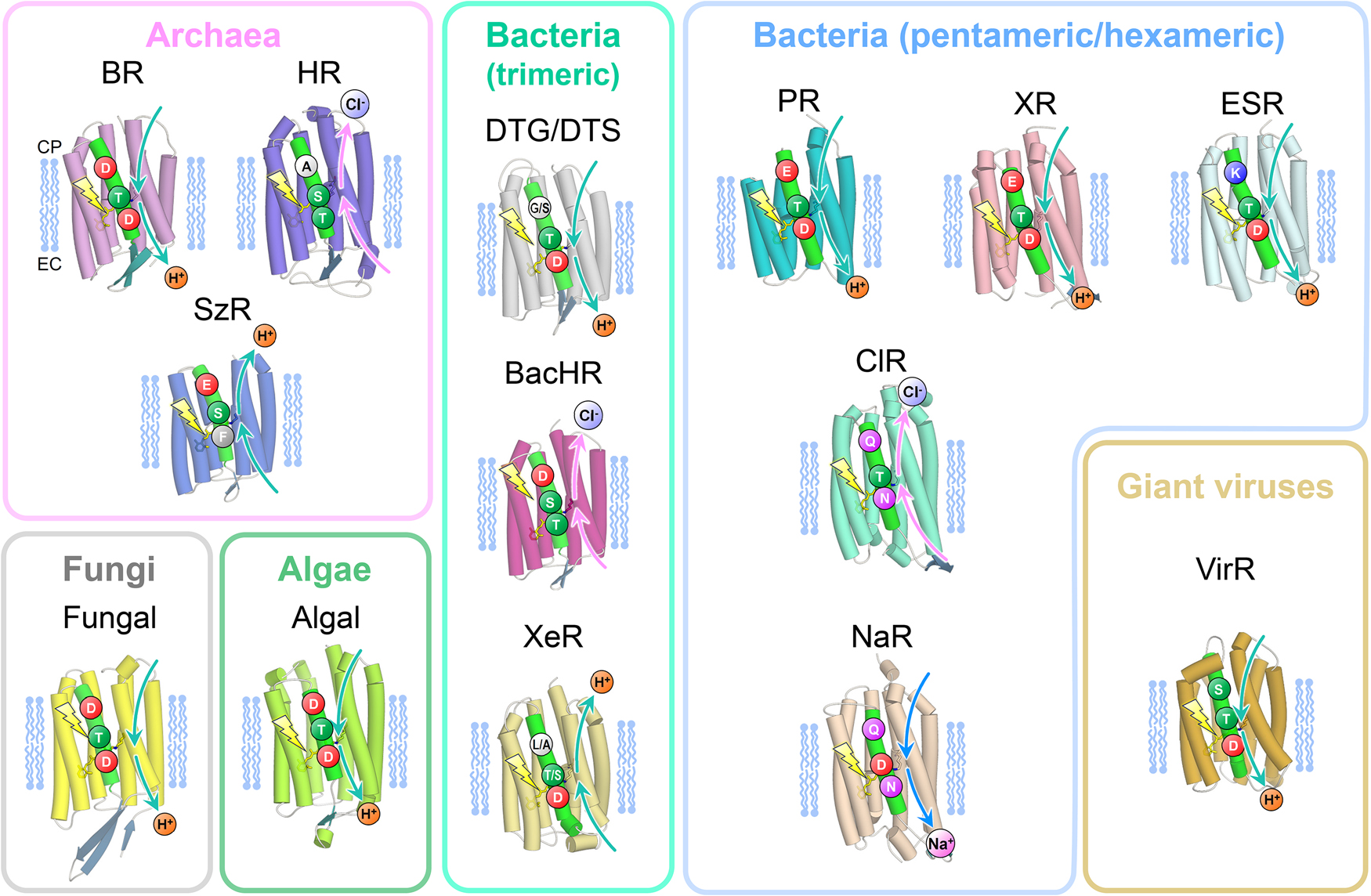
Various kinds of ion transporting rhodopsins. Three amino acid residues on the TM3 are depicted with direction of ion transport and ion types. The residues are conserved in each ion pumping rhodopsin and constitute a motif (e.g. DTD motif in BR). This figure was generously provided by Prof. Keiichi Inoue in the University of Tokyo.
Molecular mechanisms of microbial rhodopsins have been extensively studied by various kinds of biophysical methods, such as sophisticated spectroscopic techniques in UV-visible, infrared, and microwave region [26]. UV-visible spectroscopy provides specific electronic state information of the chromophores (retinal and carotenoid) not only in the ground state, but also its electronically excited state, and transiently formed intermediate states when adopting flashlight of laser with a time-resolved measurement systems. Among them, resonance Raman spectroscopy especially provides fruitful information about structural changes of the chromophores with extraordinal sensitivity, which can not be resolved by X-ray crystallography with moderate resolution (2.0–3.0 A). It is widely recognized that three dimensional atomic structures obtained by X-ray crystallography and cryogenic electron microscope (Cryo-EM) successfully established the solid bases for exploring molecular mechanisms of microbial rhodopsins. Nuclear magnetic resonance (NMR) techniques also provide valuable structural information of the chromophores and the protein moieties [27–29].
Light-induced difference infrared spectroscopy has been extensively applied to elucidating structural changes of the retinal chromophore and protein moieties, including side chains and even internal water molecules in microbial rhodopsins [30,31]. Those O–H and O–D stretching vibrations of internal water molecules were reported in many microbial rhodopsins [31–33]. It should be noted that strongly hydrogen-bonded water molecules near the PRSB contribute to storing the light energy in outward proton pumping rhodopsins [34]. By using time-resolved infrared spectroscopy, Prof. Klaus Gerwert (the first speaker of the session 5) first reported transient decrease of continuum band from water as a result of proton ejection from a protonated water cluster in the extracellular side in BR [35]. Recently, overtone and combination bands of retinal chromophore and water molecules in BR were recorded by light-induced difference infrared spectroscopy and analyzed with the aid of quantum chemical calculations considering anharmonicity of molecular vibrations [36]. Such topics were discussed by Prof. Víctor A. Lórenz-Fonfría (the fourth speaker of the session 5). Accordingly, infrared spectroscopy has advantage for analyzing transient hydrogen-bonding change of internal water molecules and side chains of amino acid residues in the protein moiety.
Resonance Raman spectroscopy is another vibrational spectroscopy, which provides irreplaceable benefit for studying the retinal chromophore in microbial rhodopsins. It should be emphasized that the conformation and configuration of the retinal chromophore in intermediate states with deprotonated Schiff base can be analyzed only by resonance Raman spectroscopy since infrared absorption of the retinal chromophore with deprotonated Schiff base is considerably weak. Making the best use of this technique, the molecular mechanism of an inward proton pump of SzR was elucidated by Prof. Yasuhisa Mizutani (the second speaker of the session 5). His research group found that the cis-trans re-isomerization takes place before re-protonation of the retinal Schiff base, which nicely explains the difference between outward and inward proton pumps [37]. Upon formation of M intermediate with the 13-cis form in SzR, the PRSB directs to the cytoplasmic side (CS) and releases a proton, then the retinal chromophore thermally isomerizes to all-trans and accepts a proton from the extracellular side (ES), which explains unidirectional inward proton pumping in SzR [37].
X-ray crystallography is a powerful tool to unveil the molecular mechanism of microbial rhodopsins in the atomic resolution. Recently, the serial femtosecond X-ray crystallography (SFX) using an X-ray free electron laser (XFEL) makes it possible to generate a molecular movie with high time and spatial resolutions. The first molecular movie was reported on BR [38]. The movements of the retinal chromophore, nearby protein moiety, and water molecules were experimentally resolved in time dependent manner. These three-dimensional information with atomic resolution enabled a theoretician to analyze the proton transfer mechanism in BR using a quantum mechanics method. Dr. Junichi Ono (the fifth speaker of the session 5) focused on the proton transfer reactions in BR and revealed hydronium (H3O+) and hydroxide (OH–) ions are involved [39–41]. Namely, in the first proton transfer from the PRSB to D85, it was proposed that a transiently formed hydroxide ion pulled a proton from the PRSB [39]. Then the second proton ejection occurred from the proton releasing group (including D204 and S193) to the extracellular surface via the Grotthus mechanism involving hydronium ions [40]. Finally, the third proton that was transferred from the proton donor D96 to the Schiff base was performed by sequentially formed proton hole (hydroxide ion) along a transiently formed water wire connecting between D96 and the Schiff base [41]. In a past literature, involvement of hydroxide ion was proposed in a hypothesis that BR is not a proton pump but a hydroxide ion pump [42]. Hydroxide ion in proton pumping mechanism in BR appears again in different context.
In contrast to proton, cations and anions have electron density which can be resolved in an electron density map obtained by X-ray crystallography or Cryo-EM. The exact location changes of a substrate ion in the unphotolyzed and the different intermediate states provide direct evidence of the ion transporting pathway. Thus, chloride and sodium pumping rhodopsins have been extensively studied with cryo-trapping and time-resolved methods. Here, one interesting report on a sodium pumping rhodopsin (NaR) from Prof. Takashi Kikukawa (the third speaker of the session 5) was introduced briefly. NaR from the bacterium Indibacter alkaliphilus (IaNaR) has been extensively studied by his group, and found IaNaR to exhibit sodium pumping activity, and the intermediate states recorded in the photocyclic reaction were similar to that of KR2 [43–46]. Two conformers of IaNaR in an asymmetric unit have been determined by X-ray crystallography. According to the presentation by Prof. Kikukawa, one conformer resembled to the unphotolyzed state of KR2, the other one exhibited distinct structure especially in the EC side. This conformer may mimic the structure formed upon release of Na+ ion to the EC side. NaR does not bind a substrate Na+ in the unphotolyzed state, which shows clear contrast to other ion-transporting rhodopsins. A proton is bound to the Schiff base in outward and inward proton pumping rhodospins, while a chloride ion is located near the PRSB in chloride-pumping rhodopsins. Cryo-trapping [47] and time-resolved [48] X-ray crystallography captured the structures binding a substrate Na+ in O intermediate states of KR2. Although there are some discrepancies between the O intermediates reported by the two groups [49], X-ray crystallography structures provide us abundant structural information for discussing the ion-transporting mechanism of NaR. The peculiarity in molecular mechanism of NaR was also discussed in a previous review [50].
Ion-transporting mechanism of membrane proteins other than microbial rhodopsins is generally complicated due to structural complexity coming from the large protein complexes (e.g. respiratory and photosynthetic systems). Microbial rhodopsin is basically composed of only one protein unit with one or two chromophores. Thus, essence of ion-transporting mechanism must be obtained from comparison of microbial rhodopsins with different directions and ion types for transportation. This brief review does not cover all ion transporting rhodopsins. There are many valuable reviews regarding this topic [1–4,22,30,31,33,34,50–53]. We hope this review helps readers understanding recent progress of ion-transporting rhodopsins in some part.
The authors thank Prof. Keiichi Inoue in the University of Tokyo for providing figures used in this review article.