2019 Volume 2 Issue 5 Pages 86-90
2019 Volume 2 Issue 5 Pages 86-90
This study proposes and evaluates a precise and labor-saving method for quantifying phthalic-acid esters (PAEs) in indoor air based on solid-phase extraction. Three different adsorbents were evaluated; i.e., two types of octadecyl silica (ODS) filter and a styrene–divinylbenzene (SDB) copolymer cartridge. Calibration curves for five PAEs [diethyl phthalate (DEP), diisobutyl phthalate, di-n-butyl phthalate (DBP), benzyl butyl phthalate (BBP), and di(2-ethylhexyl) phthalate (DEHP)] were created using an internal standard (DBP-d4). Values of the coefficient of determination (R2) indicated good linearity of the calibration curves (R2 > 0.9953). Among the three adsorbents, the SDB cartridge was easiest to handle because it can be used without cleaning and has the lowest blank value. The recovery of deuterated PAEs (DEP-d4, DBP-d4, BBP-d4, and DEHP-d4) did not differ significantly among the three adsorbents; values were consistently > 89.7% for an air volume of 2.88 m3. During simultaneous indoor air sampling, PAE concentrations were very similar for the three adsorbents. Interlaboratory validation studies were conducted in five laboratories to validate the proposed method for two PAEs (DBP and DEHP). The mean recoveries of the two PAEs added to two types of adsorbent were 91.3–99.9%, the reproducibility relative standard deviations (RSDR) were 5.1–13.1%, and the Horwitz ratio (HorRat) values were 0.31–0.79. The proposed method using solid-phase extraction with two types of adsorbents provides accurate estimates of PAEs in ambient air.
Phthalic acid esters (PAEs) are the most abundantly produced, and the most widely used plasticizers in the world. PAEs are contained in many kinds of industrial products used in indoor environments, such as polyvinyl chloride covering for floors and walls, outer casings of computer monitors and TV sets, synthetic-leather products, and electrical cables. PAEs are released from these products into the indoor environment, existing as ubiquitous pollutants. Consequently, house dust is identified as an important source of human exposure to PAEs, in addition to food and drinking water.1–3)
Although PAEs are substances with low acute and chronic toxicity, they are often mentioned as suspected endocrine disruptors, mainly because of their testicular toxicity.4–6) Since 2000–2001, the Japan Ministry of Health, Labor, and Welfare (MHLW) has set indoor guidelines for di-n-butyl phthalate (DBP) and di(2-ethylhexyl) phthalate (DEHP) based on their reproductive toxicity. Recently, the guidelines values were made more stringent, decreasing the permissible values from 220 μg/m3 to 17 μg/m3, and from 120 μg/m3 to 100 μg/m3, for DBP and DEHP, respectively.7)
MHLW also set two different standard sampling/analytical protocols for DBP and DEHP in indoor air in 2001: solid phase adsorption followed by solvent extraction or thermal desorption, and determination by a combination of gas chromatography (GC) and mass spectrometry (MS).8) Another method is described in ISO 16000-33 (2017),9) which concerns determination of PAEs in indoor air using GC-MS. In the ISO methods,9) FlorisilTM (Merck) is used as the adsorbent for the solvent extraction methods. In contrast, three other kinds of adsorbents—activated carbon, octadecyl silica (ODS), and styrene-divinylbenzene (SDB) copolymer—are recommended in Japanese standard protocol.8) FlorisilTM preparation, according to ISO 16000-33, is very time-consuming because it requires 6 h of heating at 800°C and 45 min of mixing after addition of water. On the other hand, preparation of each adsorbent according to the Japanese standard protocol is much easier and requires at most 1 h.
Our research group has reported on the determination of semi-volatile organic compounds (SVOCs) in indoor air, such as PAEs, non-PAEs plasticizers, organophosphates, and polybrominated flame retardants.10,11) In these previous studies, ODS filter was used as adsorbents in combination with quartz-fiber filter. Our recent research has shown, however, that ODS filter alone is enough for sampling PAEs in indoor air. Regarding adsorbents other than ODS, a newly developed Japanese SDB copolymer cartridge (SDB cartridge) is sufficient for SVOC sampling and has an excellent low blank value.12) In this study, to develop a simple and labor-saving method for the measurement of PAEs in indoor air, we first evaluated two kinds of adsorbents, i.e., an ODS filter and SDB cartridge, and then carried out an interlaboratory validation study.
The PAE standard and deuterated PAEs were purchased from Kanto Chemical (Tokyo, Japan). The five selected PAEs include two with existing recommended limits in indoor air (DBP and DEHP) and three which have also been detected in indoor air5,13) [diethyl phthalate (DEP), diisobutyl phthalate (DIBP), and benzyl butyl phthalate (BBP)]. DEP-3,4,5,6-d4 (DEP-d4), DBP-3,4,5,6-d4 (DBP-d4), BBP-3,4,5,6-d4 (BBP-d4), and DEHP-3,4,5,6-d4 (DEHP-d4) were selected as the internal standards. Acetone (marketed as useful for residual pesticide and PCB analysis) was purchased from Fujifilm Wako Pure Chemical Corporation (Osaka, Japan).
Air SamplingTwo types of ODS filter and an SDB cartridge were used as adsorbents. One ODS filter was an EmporeTM Disk C18 measuring 47 mm (3M Company, St. Paul, MN, USA), and the other was an ENVI™-18 DSK SPE Disk measuring 47 mm (Merck, Tokyo, Japan). Each filter was rinsed with fresh acetone five times and dried on a clean bench prior to use then installed in an aluminum holder (EMO-47, GL Sciences Inc., Tokyo, Japan). The SDB cartridge was an AERO LE Cartridge SDB400HF (GL Sciences) (Fig. 1). The SDB copolymer (mesh 30/60; 400 mg) was cleaned and packed into a glass tube (length = 21 mm; diameter = 19 mm) before shipping, so that it could be used without further cleaning. The cartridge was installed in an AERO Holder (GL Sciences) before air sampling.

Structure of the AERO LE Cartridge SDB400HF
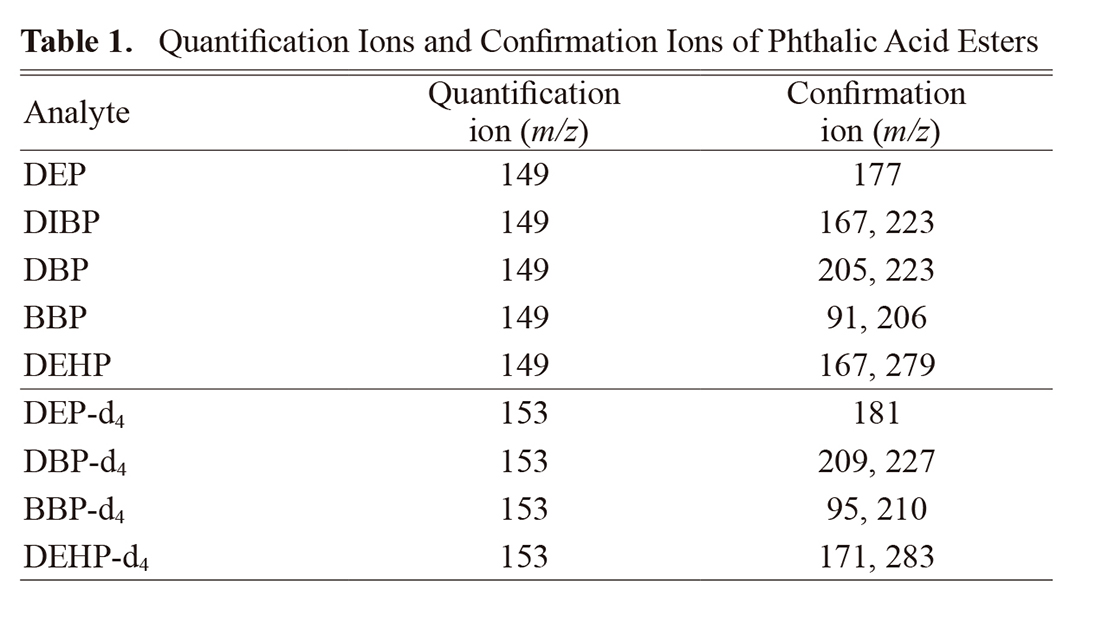
PAE analysis was performed using a Shimadzu GCMS-QP2010 instrument. A DB-1 column with an inner diameter of 15 mm × 0.25 mm and a film thickness of 0.1 μm (Agilent Technologies Japan, Ltd., Tokyo, Japan) was used as the GC analytical column. The injector was operated in splitless mode at a temperature of 280°C. The GC oven temperature was held at 80°C for 2 min, increased at 8°C/min to 210°C and maintained for 5 min, then increased at 20°C/min to 250°C and maintained for 5 min. The selected-ion monitoring mode was used for analysis: two or three ions were selected per target analyte (Table 1). DEP-d4, DBP-d4, BBP-d4, and DEHP-d4 were monitored as the internal standards.
 Quality Assurance/Quality Control
Quality Assurance/Quality Control
The limit of detection (LOD) was defined as the absolute amount of an analyte that yields S/N = 5. When blank values exceed five times the value of noise, the LOD was defined as double the blank values. The method detection limits (MDLs) of the air samples were calculated by the LODs, volume of the extract, and air sample volume.
Method ValidationRecovery tests and simultaneous indoor air sampling were performed using the three adsorbents. For the recovery test, each adsorbent was spiked with 0.5 μg of DEP-d4, DBP-d4, BBP-d4, and DEHP-d4. Then, indoor air was passed through at a flow rate of 2 L/min or 10 L/min for 24 h (corresponding to an air volume of 2.88 m3 or 14.4 m3; n = 3). Simultaneous indoor air sampling was conducted using the three adsorbents at a flow rate of 2 L/min for 24 h (n = 3). After air was passed through, the adsorbents were extracted by ultrasonication for 10 min using 10 mL of acetone. A 5 mL aliquot of the extract was then concentrated under nitrogen to 0.5 mL. The internal standards that were added to the 5 mL extract before concentration were Fuluoranthen-d10 for the recovery test and DBP-d4 for the simultaneous indoor air sampling. After GC-MS analysis, the percentage recoveries and indoor air concentrations were calculated.
Interlaboratory Validation StudyFive laboratories participated in the interlaboratory validation study: the Tokyo Metropolitan Institute of Public Health (Shinjuku-ku, Tokyo), two laboratories of the National Institute of Health Sciences (Kawasaki, Kanagawa), the Hokkaido Institute of Public Health (Sapporo, Hokkaido), and the Kanagawa Prefectural Institute of Public Health (Chigasaki, Kanagawa). Each laboratory was assigned an identification code of A–E in no specific order.
Interlaboratory validation studies were conducted for two PAEs (DBP and DEHP), whose indoor air recommended values have been defined by MHLW. As validation parameters, the accuracy, precision, and reliability were evaluated in all five laboratories. ODS filters (3M Company) were cleaned by immersion with acetone (20 mL/disk) five times and dried on a clean bench prior to use. For the blank test, ODS filters washed with acetone by the above-mentioned clean-up method were stored in an acetone-washed collinear test tube using tweezers, and the stopper was wound with aluminum foil and Teflon tape then sealed to avoid contamination with PAEs. The purchased SDB cartridge was used as a sample for the blank test with no further processing. For the recovery evaluation test, 4 μg each of DBP and DEHP were added to the two adsorbents; i.e., pre-cleaned ODS filters and SDB cartridge, then transported by courier to each participating laboratory. In the laboratories, the two PAEs were extracted from the two adsorbents (n = 5) using 5 mL of acetone and analyzed by GC-MS using the analytical method of each laboratory.
The relative standard deviation (RSD: SD/mean) was used to assess fluctuations in the data relative to the data mean. The repeatability, or within-laboratory reproducibility, measured by the relative standard deviation (RSDr) and the between-laboratory reproducibility, measured by the relative standard deviation (RSDR) were used as indicators of precision. The precision results were then compared with the Horwitz predicted interlaboratory precision by calculating the Horwitz ratio (HorRat).14) The HorRat value14) is the ratio of the RSDR calculated from the data of each laboratory to the RSDR predicted from the Horwitz equation (PRSDR):
HorRat = RSDR/PRSDR
Under reproducibility conditions, acceptable HorRat values are between 0.5 and 2. When the concentration of the chemical substance to be analyzed is 10-7 or more, the PRSDR (%) is calculated by the following formula,
PRSDR (%) = 2 (1 – 0.5 log C)
where C is the commonly measured concentration of the analyte in the sample, expressed as a mass fraction.
The calibration curves of target PAEs were made using a six-point standard series, 0.2, 0.5, 1.0, 2.0, 5.0, and 10.0 μg/mL. The three kinds of internal standard, DBP-d4, BBP-d4, and DEHP-d4, were added to each of the calibration standards.
To choose a proper internal standard based on the linearity of the calibration curves for five analytes, the calibration curves were made using each of the three internal standards, and the coefficients of determination (R2) were compared with each other (Table 2). A good linearity (R2 > 0.98) was obtained for all analytes when using the deuterium-labeled PAEs as internal standards. The R2 values were in the range of 0.9953–0.9997, 0.9900–0.9993, and 0.9891–0.9992 for DBP-d4, BBP-d4, and DEHP-d4, respectively. Thus, DBP-d4 was identified as the most suitable internal standard in view of the linearity of the calibration curves for PAEs.

In the blank test, DEP, DBP, and DEHP were detected from all adsorbents (Table 3), while the two other target PAEs—DIBP and BBP—were not detected. The range of blank values were as follows; DEP, 1.3–2.6 ng; DBP, 10.1–30.1 ng; and DEHP, 13.7–32.7 ng, indicating that SDB cartridge was the most superior in view of the background levels of PAEs among the three adsorbents examined.

Table 4 summarizes the MDLs of each PAE. The MDLs of PAEs were in the range of 2.0–45.0 ng/m3 at the air sampling volume of 2.88 m3, while MDL ranges of 0.40–9.0 ng/m3 were obtained when the larger sampling volume (14.4 m3) was applied.
 Method Validation
Method Validation
Table 5 shows the recovery of deuterated PAEs spiked to the adsorbents, after passing through indoor air (2.88 m3 or 14.4 m3). The percentage recoveries were in the range 89.7–95.5% at the air sampling volume of 2.88 m3 and 85.9–100% at the air sampling volumes of 14.4 m3, indicating that the deuterated PAEs were almost quantitatively recovered from any of the adsorbents.

To test the equivalency of the three adsorbents, indoor air was sampled simultaneously using ODS filter (3M), ODS filter (Merck), and SDB cartridge, and analyzed by GC-MS. The PAE concentrations in the indoor air sample ranged from 16.9 to 17.1 ng/m3, 7.0 to 7.2 ng/m3, 90.0 to 96.2 ng/m3, and 64.9 to 72.3 ng/m3 for DEP, DIBP, DBP, and DEHP, respectively (Table 6). These results demonstrated that the three adsorbents were almost compatible with each other.
 Interlaboratory Validation Study
Interlaboratory Validation Study
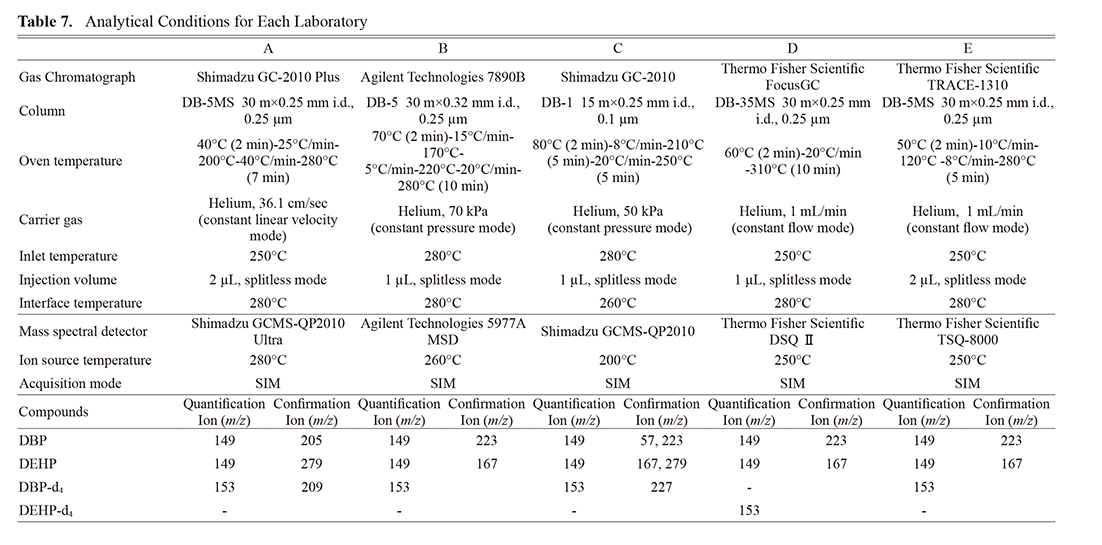
To establish the method performance characteristics, an interlaboratory validation study was carried out. Five different laboratories participated in the study, and analytical instruments and conditions in collaborating laboratories were summarized in Table 7.
Selectivity was evaluated by GC-MS analysis of a blank test, verifying the absence of signal interference in all collaborative laboratories (data not shown).
Accuracy, which was determined by the recovery study, was evaluated by preparing two kinds of adsorbents (ODS filter (3M) and SDB cartridge) spiked with 4 μg of DBP and DEHP. Table 8 shows the results of intra- (within) and inter- (between) reproducibility in the recovery test.
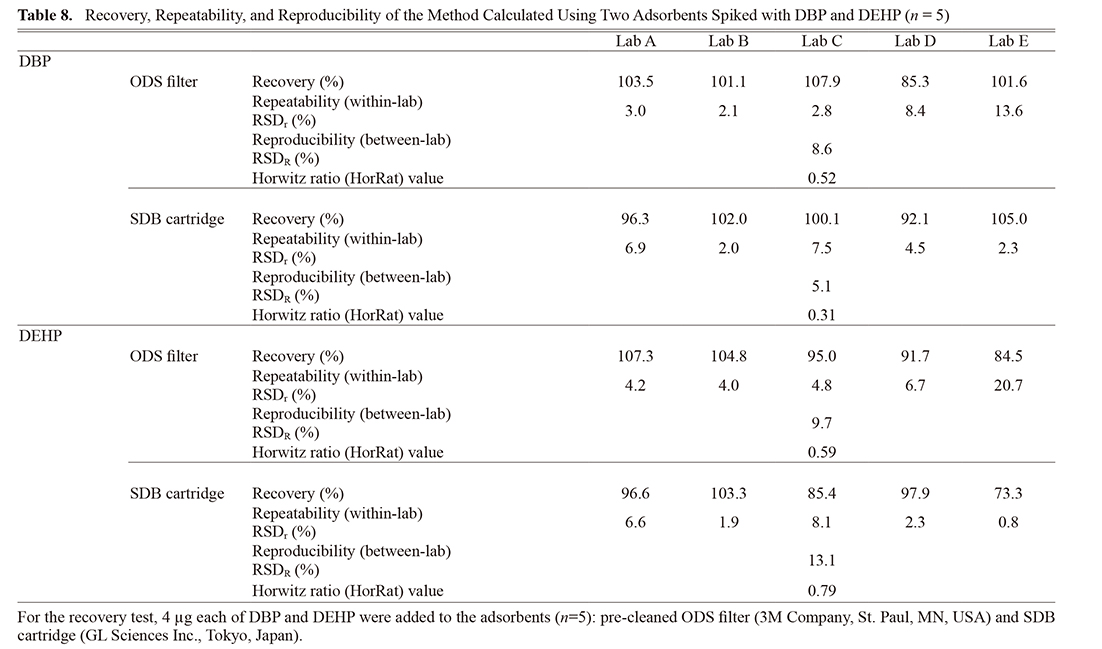
In the case of DBP, the recoveries were between 85.3 and 107.9% (ODS filter), and 92.1 and 105.0% (SDB cartridge). In the case of DEHP, the recoveries were between 84.5 and 107.3% (ODS filter), and 73.3 and 103.3% (SDB cartridge).
The within-laboratory reproducibility, relative standard deviations (RSDr), of DBP were 2.1–13.6% for ODS filter and 2.0–7.5% for SDB cartridge. RSDr of DEHP were 4.0–20.7% for ODS filter and 0.8–8.1% for SDB cartridge. On the other hand, the interlaboratory reproducibility, relative standard deviation (RSDR), of DBP was 8.6% for ODS filter and 5.1% for SDB cartridge, while RSDR of DEHP was 9.7% for ODS filter and 13.1% for SDB cartridge.
The interlaboratory reproducibility (RSDR) values were compared with the predicted levels of precision obtained from the Horwitz equation. The predicted RSDR was calculated to be 16.55%, according to the Horwitz equation. The HorRat value—the ratio of RSDR (measured) to the predicted RSDR (Horwitz)—gives a comparison between the actual precision and the precision predicted by the Horwitz equation. The HorRat values ranged from 0.31 to 0.79 (Table 8).
The results of this study consistently demonstrate that all three adsorbents examined were useful for indoor air PAE measurement. Among the adsorbents, SDB cartridge is the easiest to handle because it can be used without cleaning and has the lowest blank level. In the simultaneous indoor air sampling and interlaboratory study, DBP-d4 alone was used as an internal standard. Although use of multiple internal standards is recommended in ISO 16000-33,9) the use of a single internal standard is less laborious in quantitative work. Comparison of the linearity of the calibration curves for the three internal standards indicated that DBP-d4 had the highest R2, hence it was selected as the internal standard in this study. However, when using SDB as an adsorbent, it is better to add DEHP-d4 to test samples as an internal standard, because the matrix effect tends to interfere the determination of DEHP.
Interlaboratory validation studies were conducted for two PAEs, DBP and DEHP. As validation parameters, the accuracy, precision, and reliability were evaluated in five laboratories. In the Hazardous Air Pollutant Measurement Method Manual by the Ministry of the Environment (revised in March 2011),15) the “Target Quantitative Lower Limit” has been introduced as a criterion for judging the acceptability of the lower limit of quantification and operation blank values. The target lower limit of quantification is determined in light of the purpose of measurement, etc., but in principle it is 1/10 of the environmental standard and guideline values for substances of interest. In indoor guidelines, the threshold values for DBP and DEHP are 17 μg/m3 and 100 μg/m3, respectively.7) In the recovery test for the purpose of evaluating accuracy, an indoor PAEs concentration was assumed to be 1 μg/m3. This level is well below the threshold values, 1/17 for DBP and 1/100 for DEHP. When indoor air, containing PAEs at a concentration of 1 μg/m3, is collected at a flow rate of 3 L/min for 24 h, about 4 μg of each PAE is supposed to be collected on the absorbent. Taking these considerations into account, DBP and DEHP were spiked at the level of 4 μg to two kinds of adsorbents. As a consequence, good reproducibility was demonstrated in the interlaboratory validation studies. The calculated HorRat values can be used as a performance parameter, which indicate the acceptability of the precision of the method. A HorRat value of <2 usually indicates satisfactory reproducibility of the method. In this study, all HorRat values for PAEs were lower than 2, indicating that the measurement of DBP and DEHP by the developed method in this study was validated by an interlaboratory test in accordance with internationally harmonized protocol.
Conclusively, in this study, a reliable quantitation method for determining DBP and DEHP in indoor air was developed and fully validated. This labor-saving method will enable nationwide surveys of indoor air quality.
This study was supported in part by a grant from Research on Risk of Chemical Substances, Health, Labour and Welfare Policy Research Grants, Ministry of Health, Labour and Welfare.