2020 年 3 巻 4 号 p. 126-129
2020 年 3 巻 4 号 p. 126-129
Melanocytes increase melanin production upon exposure to ultraviolet rays (UV) as a defense mechanism. Melanin synthesis in melanocytes is regulated by bioactive factors produced and secreted by keratinocytes. In addition, melanocytes transfer biosynthesized melanin to keratinocytes, causing skin pigmentation. During the pigmentation process, melanocytes communicate with surrounding cells. However, the molecules involved in this cell-to-cell communication, particularly in melanocytes, are poorly understood. In this study, we focused on changes in cell membrane protein expression following exposure of melanocytes to UV irradiation. After mouse melanoma B16 cells were exposed to UV irradiation, alterations in the expression of cell membrane proteins were analyzed using peptide mass fingerprinting. We identified the membrane protein vinculin from proteins with enhanced expression. Western blot results confirmed UV exposure increased vinculin in cell membranes. In contrast, there was no change in vinculin levels in whole cell extracts. Furthermore, we observed no variation in mRNA expression levels using real time PCR. Melanocytes exposed to UV enhanced vinculin migration to cell membranes without altering expression levels. We suggest vinculin involved in the cellular responses of melanocytes and keratinocytes.
The rays of the sun that reach the Earth are composed of ultraviolet, visible light, and infrared wavelengths. Of these, UVA (320-400 nm) and UVB (290-320 nm) are the wavelengths that have the most influence on skin homeostasis. UVA and UVB are energy dense and induce various inflammatory responses in human skin after exposure occurs.1,2) Typical skin responses to UVB exposure include erythema and sunburn, followed by pigmentation.3) When sunburn occurs, reactive oxygen species (ROS) harm cells in the epidermis, and in particular, keratinocytes are damaged by the lipid oxidation that occurs in the cell membrane.4) In addition, UVB can cause thymine dimers to form in intracellular DNA.5,6) Thymine dimers interfere with DNA replication and transcription, leading to mutations or cell death. Although UVA contains less energy than UVB, UVA also causes skin pigmentation and other responses.
Skin reactions following UV exposure aim to protect the organism from UV radiation, and the development of skin pigmentation is a typical response.3) Pigmentation after UV exposure follows various processes. For example, the hyperpigmentation induced by UV exposure involves melanocytes producing melanin, which is then transported to keratinocytes.7–9) During this process, various bioactive factors are released by the surrounding keratinocytes to promote melanin production in melanocytes.10) There are numerous bioactive factors promoting melanin production, including bioactive peptides, such as melanocyte-stimulating hormone (α-MSH, β-MSH and γ-MSH), adrenocorticotropic hormone (ACTH), β-endorphin (β-END) and endothelin-1 (ET-1); as well as growth factors, such as stem cell growth factor (SCF), basic fibroblast growth factor (bFGF), granulocyte-macrophage stimulating factor (GM-CSF), nerve growth factor (NGF), leukemia inhibitory factor (LIF) and hepatocyte growth factor (HGF); and lipid mediators, such as prostaglandin E2 (PGE2), that are released by cells such as keratinocytes. Despite this, the mechanisms for transporting the melanin produced by melanocytes to keratinocytes are still unclear. In this study, we searched for proteins whose expression fluctuated in the cell membrane of melanocytes after exposure to UV light and used the observed changes to identify molecules involved in the transportation of melanin from melanocytes to keratinocytes.
The mouse melanoma cell line B16 was supplied by the Health Science Research Resources Bank. B16 cells were cultured in DMEM medium (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS; Moretate Biotech, Bulimba, Australia).
Exposure of Cells to UVInitially, 106 cells were seeded in a 10 cm petri dish. After 24 h of culturing, cells were exposed to UV at 1 mJ cm-2 using an 11FL-40SE lamp (Toshiba, Tokyo, Japan). We collected the cells 24 h after the UV irradiation using a scraper. The collected cells were centrifuged at 1,000 × g at 4°C for 30 min, and the resulting pellet was collected as the cell membrane fraction. The cell membrane fraction was lysed with RIPA buffer, then centrifuged at 10,000 × g at 4°C for 30 min. The supernatants were stored at −80°C for SDS-PAGE and Western blot analysis.
SDS-PAGECell membrane samples (10 μg) were reduced with mercaptoethanol at 95°C for 10 min, then separated on a 12% polyacrylamide gel. Proteins were stained with Coomassie Brilliant Blue-G50 stain solution.
Peptide Mass Fingerprinting AnalysisFor bands detected with Coomassie Brilliant Blue-G50, bands with expression variation were cut and incubated with 30% acetonitrile in 0.1% trifluoroacetic acid until the color disappeared. Proteins underwent reductive alkylation with dithiothreitol and monoiodoacetic acid. The modified proteins were digested with TPCK-trypsin (Sigma-Aldrich, St. Louis, MO, USA) in 100 mM ammonium bicarbonate at 37°C overnight. After enzymatic digestion, the resultant peptides were extracted with 50% acetonitrile in 0.1% trifluoroacetic acid. For desalting, extracts were loaded on a ZipTipC18 peptide tip (Millipore, Billerica, MA, USA) and eluted with 60% acetonitrile in 0.1% trifluoroacetic acid. The eluent was mixed with a saturated α-cyano-4-hydroxyceinamic acid (α-CHCA) (Sigma-Aldrich) in 50% acetonitrile in 0.1% trifluoroacetic acid and applied to a target plate to dry. Mass spectrometry experiments were performed on a Bruker Autoflex (Bruker Daltonics, Bremen, Germany). We used the program Mascot (Matrix Science, London, UK) for database searches.
Western Blot AnalysisProteins were blotted on a nitrocellulose membrane (Protran BA85, GE Healthcare UK Ltd., England) in semi-dry blot system (NA-1512S, Nihon Eido, Japan). Nitrocellulose membranes were blocked with 2% skim milk. Blocked membranes were incubated with rabbit anti-vinculin antibody (1:1000; Bethyl Laboratories, Inc., Montgomery, TX, USA), followed by horseradish peroxidase (HRP)-conjugated anti-rabbit IgG goat antibody (1:10,000; Biosource, Camarillo, CA, USA). Protein bands were subsequently visualized by an ImmunoStar LD with a Luminograph (Atto, Japan). We measured band intensity using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Expression of Vinculin mRNAWe assessed vinculin mRNA expression using real time-PCR (RT-PCR) after cells were exposed to UV irradiation. B16 cells were seeded at a density of 105 cells/well in a 6-well tissue culture plate and incubated overnight. Cells were irradiated with UVB and cultured for a further 24 h.
Total RNA was extracted from melanocytes with TriPure Isolation Reagent (Roche Life Science, Upper Bavaria, Germany). Genomic DNA was removed with DNase I. We synthesized cDNA using ReverTra Ace (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. Specific primers were designed to amplify mouse vinculin (5’-CTTCGATGAGGCTGAGGTTC -3’ and 5’-TGGTGAGTCAACTCCTGCTG -3’), and mouse β-actin (5’-TGACAGGATGCAGAAGGAGA -3’ and 5’- CATCTGCTGGAAGGTGGAC -3’). We performed RT-PCR using StepOne (Applied Biosystems, Massachusetts, USA). Samples were cycled with the following conditions: 1 cycle of 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The Ct values for the samples were normalized to that of β-actin and the relative expression was calculated using the comparative Ct method.
ImmunocytochemistryB16 cells were plated on culture slides (FALCON, USA). After incubating for 24 h, cells were fixed with 4% paraformaldehyde for 10 min. After being blocked with normal goat serum (1:50) for 30 min at room temperature, cells were incubated with rabbit anti-vinculin antibody (1:100; Bethyl Laboratories, Inc., Montgomery, TX) overnight at 4°C, followed by a secondary Hilyte488-conjugated anti-rabbit goat antibody (1:500; BioSource International, Camarillo, CA) for 1 h at room temperature. Cells were then stained for actin filaments with rhodamine-conjugated phalloidin for 1 h at room temperature and stained for nucleic acid with Hoechst 33342. Fluorescence was observed with a fluorescence microscope (BZ-X700, Keyence Co. Ltd., Japan).
We extracted cell membrane proteins after UV exposure and separated the proteins using SDS-PAGE (Fig. 1-A). We isolated bands representing separated proteins that showed variable expression and analyzed these proteins by peptide mass fingerprinting with TPCK-trypsin. Mouse vinculin was identified as a protein with increased expression after UVB-irradiation (Fig. 1-B, C).
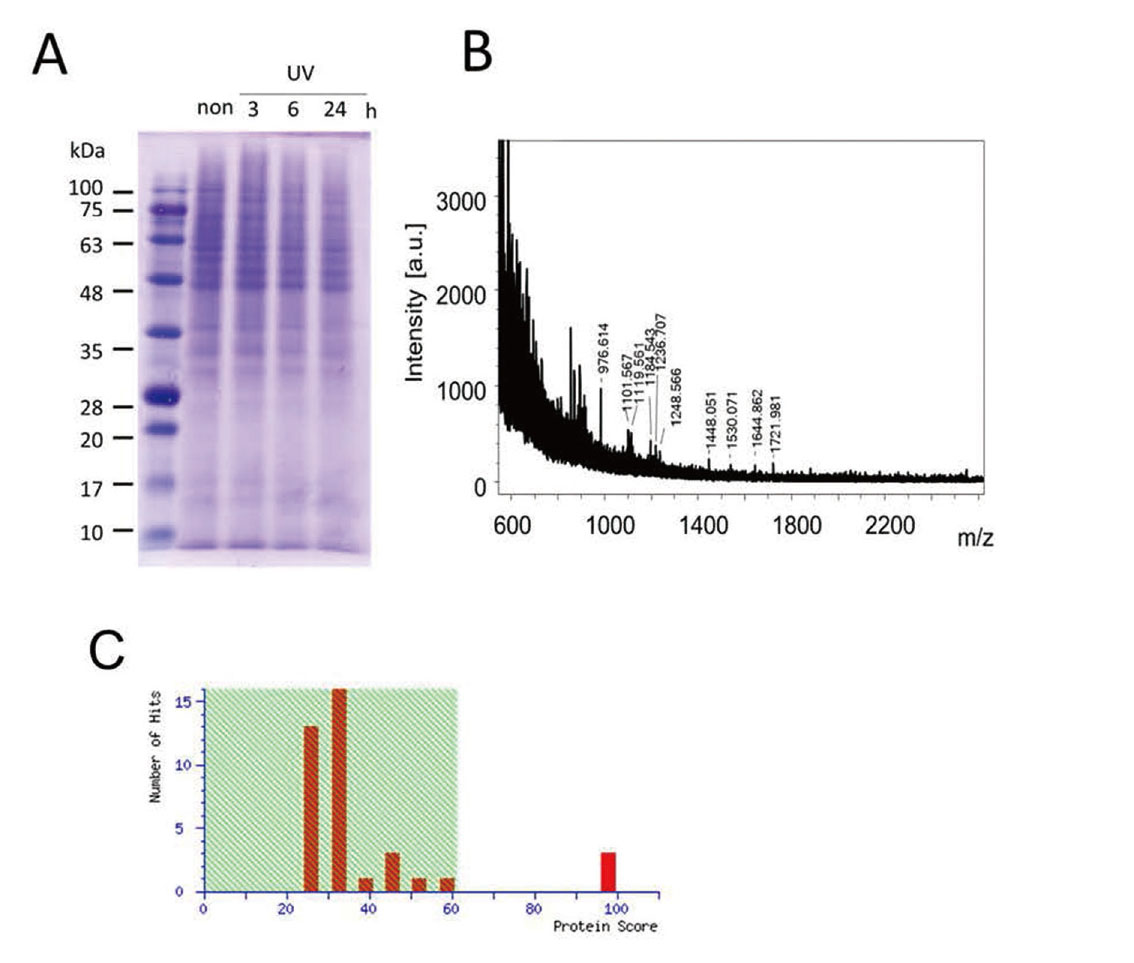
The Identification of Vinculin Among Proteins in Melanocytes that Show Variable Expression After UV Exposure
(A) SDS-PAGE for proteins after UVB irradiation. (B) Spectra of MALDI-TOF mass spectrometry for the protein that increased after UVB exposure. (C) Identification results from the MASCOT search showing the identification of mouse vinculin.
Our RT-PCR analysis showed UV exposure did not alter the expression of vinculin mRNA (Fig. 2). We then analyzed vinculin protein expression by Western blotting using whole cell or cell membrane fraction samples prepared from melanin-producing cells after UV exposure. When applied to whole cells, the UV exposure did not alter overall vinculin protein levels (Fig. 3-B, D) but increased the level of vinculin present in the cell membrane (Fig. 3-A, C). Vimentin expression increased more than 3-fold 3 h after UV exposure, and was then maintained at high levels for more than 24 h. β-actin levels did not differ among these cells. These results suggest that UV exposure does not alter total vinculin protein in cells, but rather increases the amount of vinculin that binds to the cell membrane. To investigate vinculin translocation to the membrane after UV irradiation, we examined the intracellular distribution of vinculin using immunostaining. Vinculins are intracellular lining proteins and are the starting point for actin filaments in the cell (Fig. 3-E, F). Therefore, we stained actin filaments with rhodamine-labeled phalloidin and observed the fluorescence from the double staining of vinculin and actin. We found that in cells prior to UV irradiation, vinculin proteins were widely distributed in the cytoplasm, with few vinculin proteins colocalizing with actin fibrils. Conversely, upon exposure to UV light, vinculin was redistributed to the tips of cell protrusions and colocalized with actin filaments, from which actin fibers extended toward the cell.

Analysis of Vinculin mRNA by Real-time PCR in Melanocytes After UVB Irradiation
The comparison of mRNA expression was evaluated by the ddCt method. Bars indicate means ± SEM (n = 3).

Distribution of Vinculin in Melanocytes After UVB Irradiation
Western blot analysis for vinculin in the cell membrane (A) and whole cell extract (B). The intensity ratios of vinculin in the cell membrane (C) and whole cell extract (D) were measured by densitometry. Data represent means ± SEM (n = 3 vs. 0 h). Immunohistochemistry of vinculin in melanocytes treated with UVB irradiation (F) and without UVB irradiation (E). Vinculin is stained with Hylite-488 (green), actin filament is stained with rhodamine conjugated to phalloidin (red), and nuclei are stained with Hoechst (blue). Scale bar: 50 μm.
Intense UV exposure causes tremendous damage to organisms, which has resulted in organisms being equipped with various mechanisms to protect against UV rays. Melanogenesis of the skin is the major defense mechanism against UV light and is regulated by melanin-producing cells. By investigating changes in melanin-producing cells after exposure to UV light and focusing on cell membrane proteins, we found that vinculin transitions to the cell membrane after UV exposure. Vinculin is an adapter protein and is one of the cell membrane lining proteins comprising the machinery necessary for cell adhesion.11) Vinculins are expressed in various tissues and organs. In addition, they are present at focal adhesions,12) which are binding devices for cell-substrate adhesion, as well as at adhesive junctions, which are binding devices for cell-cell adhesion.13) Exposing melanocytes to UV light has been previous shown to increase the number of adhesive junctions between melanocytes and keratinocytes.14)
Melanin produced by melanocytes forms melanosomes, which are absorbed by keratinocytes.15) There are various possible mechanisms to transport melanosomes to keratinocytes and an increase in the adhesive junctions of melanocytes to keratinocytes may play an important role in improving melanosome transport efficiency. The increased localization of vinculin in the plasma membrane led to more adhesion points between cells, and thus vinculin may have contributed to the increase in adhesion points on keratinocytes seen previously.
Localized vinculin in the plasma membrane may also improve the transport efficiency of melanosomes to keratinocytes in other ways. After UV exposure, melanocytes produce melanin at the periphery of the nucleus, and melanosomes mature in this perinuclear region. The motor proteins kinesin and dynein are found in melanosome membranes and melanosomes are transported in an energy-dependent manner along microtubules rails to the cell membrane periphery.8,9) Kinesin and dynein contribute to melanosome migration from the nucleus to the plasma membrane, while kinesin and dynein contribute to melanosome migration from the plasma membrane to the nucleus.8) Melanosomes transported to the cell membrane periphery are subsequently passed to actin fibers, which act as new transport rails, and are transported closer to the cell membrane by the driving force of myosin-Va, an actin-dependent motor protein, which leads to the accumulation of melanosomes at the dendritic ends.9) Conversely, if melanosomes are not transferred from microtubules to actin fibers, melanosomes are transported back to the perinuclear region, causing an aggregation of melanosomes at the periphery of the nucleus. Therefore, the smooth migration of melanosomes from microtubules to actin fibers is essential for skin pigmentation. Vinculin allows the extension of actin fibers from the cell membrane into the cell cytoplasm.16) Therefore, the migration of vinculin to the cell membrane after UV exposure can be thought to provide a portion of the transportation path to keratinocytes, with tubulin facilitating efficient melanosome migration onto actin fibers. Thus, the membrane localization of vinculin after UV irradiation is expected to contribute to the increase in melanosome accumulation at the membrane.
In addition, vinculin contributes to the adhesion strength with the surrounding cells and extracellular substrates, exerting a physical force on the surroundings.17,18) Vinculin in cell membranes regulates cell adhesion and extension, and when the amount of vinculin is reduced, cells cannot attach and cell extension fails due to fewer stress fibers. In addition, intracellular tension affects the binding of vinculin to talins in adhesive plaques,17) as well as the binding of vinculin to α-catenin at adherence junctions.18) These results suggest that vinculin is involved in mechanotransduction, in which mechanical forces are converted into chemical signals. Therefore, it is likely that vinculin migration to the cell membrane regulates function via a physical force stimulation provided to keratinocytes. In particular, the binding of foreign substances to the membrane surface of keratinocytes and their stimulation triggers a phagocytic reaction in keratinocytes.19) Together, these observations suggest the mechanical forces provided by vinculin at the adhesion points between melanocytes and keratinocytes may facilitate the phagocytic response of keratinocytes. The vinculin migration to the cell membrane of melanocytes after exposure to UV radiation supports the successful migration of melanosomes to the cell membrane; the subsequent transport of melanosomes into keratinocytes may also enhanced by the mechanical forces provided by vinculin, which stimulate keratinocyte phagocytosis, and thus vinculin may act in multiple steps in the pigmentation-based UV protection mechanism.
Vinculin migration was previously thought to be a molecular process involved in the migration of melanosomes generated by the exposure of keratinocytes to UV. In this study, we have shown that the exposure of melanocytes to UV promotes vinculin migration to the cell membrane. This indicates that vinculin is an important molecule involved in hyperpigmentation after UV exposure.
This work was supported in part by the MEXT KAKENHI Grant Number 17K18070.
Conflict of interestThe authors declare no conflict of interest.