2020 年 3 巻 4 号 p. 130-137
2020 年 3 巻 4 号 p. 130-137
Spinal muscular atrophy (SMA) is a progressive neuromuscular disease, associated with motoneuron loss and muscle wasting. Numerous SMA-causative mutations have been reported in survival motor neuron (SMN) gene(s); however, the pathogenic mechanism underlying SMA remains unclear. In the present study, we showed that SMN modulates the expression of transcription factor EB (TFEB), a master regulator of lysosomal genes that plays a key role in lysosome function, autophagy, and the mammalian target of rapamycin (mTOR) signaling pathway. The transfection of small interfering RNA (siRNA) targeting SMN caused a reduction in TFEB expression in the motoneuron-like NSC-34 cell line. In differentiated NSC-34 cells, either SMN or TFEB knockdown resulted in reduced lysosomes at neurites and the atypical accumulation of swollen and enlarged lysosomes in cell bodies. SMN knockdown caused the reduced expression of lysosome-related genes, resulting in the decline of lysosomal degradation and increased autophagic flux. These SMN-depletion-induced aberrations in lysosomes and autophagy could be rescued by the exogenous expression of TFEB. Furthermore, SMN depletion in NSC-34 cells resulted in the decreased phosphorylation of mTOR and its downstream signals. Finally, SMA transgenic mice exhibited reduced TFEB and lysosomal protein expression and the inactivation of mTOR signaling in the lumbar spinal cord at postnatal day 11, compared with their counterparts. These findings indicated that SMN regulates lysosomal gene expression and functions by altering TFEB expression in motoneurons. The targeting of lysosomes might represent a new strategy for the treatment of SMA.
Spinal muscular atrophy (SMA) is an autosomal recessive, neurodegenerative disorder, characterized by the selective loss of motoneurons in the spinal cord.1) Numerous deletion and point mutations in survival motor neuron gene-1 (SMN1) have been reported, after it was identified as a causative gene for SMA.2) Human genomes contain a copy gene, SMN2 (over 99% identical to SMN1), which produces less than 10% functional, full-length SMN protein, due to a point mutation in exon 7.3,4) The copy number of SMN2 has been inversely correlated with the severity of pathological symptoms and defects in neuromuscular junctions (NMJs).2) The well-described functions of SMN include the biogenesis of spliceosomal small nuclear ribonucleoprotein particles (snRNPs) and the axonal delivery of RNA.5,6) SMN protein is degraded by the ubiquitin-proteasome system and autophagy.7,8) However, the role played by SMN in lysosomes has been less well-investigated.
Lysosomes are intracellular, acidic organelles that play a central role in the degradation of endocytosed extracellular materials and cell surface receptors and nutrient metabolism.9,10) Transcription factor EB (TFEB) is a master regulator of the coordinated lysosomal expression and regulation (CLEAR) network, which regulates the transcription of genes associated with lysosomes and autophagy.11) The interaction between TFEB and mammalian target of rapamycin (mTOR) plays an essential role in the regulation of catabolic processes and autophagy under starvation conditions.12,13) Amino acid signaling promotes the recruitment of mTOR complex 1 to the lysosomal surface, resulting in the activation of the mTOR downstream pathway, such as ribosomal protein S6 kinase beta-1 (also known as p70S6 kinase, p70S6K) and S6 ribosomal protein (S6RP), which are involved in protein synthesis.14) The dysregulation of mTOR has been shown to be involved in SMA pathophysiology.15) In addition, TFEB has been shown to be selectively reduced in the brains of amyotrophic lateral sclerosis (ALS) and Alzheimer's disease patients.16) Moreover, TFEB function has been reported to be disrupted by the polyglutamine-expanded androgen receptor, which causes spinal and bulbar muscular atrophy (SBMA).17) These findings indicate the important role played by TFEB in motoneurons and suggest a possible link between TFEB dysfunction and neurodegenerative diseases, including SMA.
Here, we investigated the link between TFEB and SMN, using in vitro NSC-34 cell cultures and an in vivo SMA model animal. SMN knockdown (KD) reduced TFEB expression, at both the mRNA and protein levels. Concomitantly, SMN-depleted cells displayed reduced lysosome-related gene expression, lysosome dysfunction, and increased autophagy influx. Furthermore, SMN depletion resulted in the decreased phosphorylation of mTOR and its downstream effectors, p70S6K and S6RP. Finally, reduced TFEB and lysosomal protein expression and the inactivation of mTOR signaling were identified in the spinal tissue of SMA transgenic mice. These findings provided new insights into the roles played by TFEB and lysosomes in SMA etiology.
The primary antibodies used were as follows; SMN [mouse, BD Biosciences, Franklin Lakes, NJ, USA, Cat# 610647, 1:10,000 for immunoblotting (IB)], TFEB [rabbit, Proteintech, Rosemont, IL, USA, Cat# 13372-1-AP, 1:5,000 for IB, 1:200 for immunofluorescence (IF)], β-actin (mouse, Sigma-Aldrich, St. Louis, MO, USA, Cat# A2228, 1:100,000 for IB), Lamp1 (rat, Abcam, Cambridge, UK, Cat# 1b25245, 1:10,000 for IB, 1:400 for IF), β-Tubulin III (rabbit, GeneTex Inc., Irvine, CA, USA, Cat# GTX130245, 1:2,000 for IF), cathepsin D (goat, R&D Systems, Minneapolis, MN, USA, Cat# AF1029-SP, 1:5,000 for IB), microtubule-associated protein light chain 3 (LC3B, rabbit, GeneTex, Cat# GTX127375, 1:10,000 for IB), p-mTOR (Ser 2448, rabbit, Cell Signaling Technology, Danvers, MA, USA, Cat# 2971S, 1:10,000 for IB), mTOR (rabbit, Cell Signaling Technology, Cat# 2972S, 1:10,000 for IB), p-p70S6K (rabbit, Cell Signaling Technology, Cat# 9234S, 1:500 for IB), p70S6K (rabbit, Cell Signaling Technology, Cat# 9202S, 1:1,000 for IB), p-S6RP (rabbit, Cell Signaling Technology, Cat# 4856S, 1:1,000 for IB), S6RP (rabbit, Cell Signaling Technology, Cat# 2317S, 1:2,000 for IB), green fluorescent protein (GFP, rabbit, MBL Life Science, Aichi, Japan,, 1:50,000 for IB).
Alexa-dye conjugated secondary antibodies were purchased from Thermo Fisher Scientific (Waltham, MA, USA, 1:400 for IF). Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Thermo Fisher Scientific (1:10,000 for IB). Small interfering RNAs (siRNAs) targeting Smn (siSMN#1, Cat# Smn1MSS209213; siSMN#2, Cat# Smn1MSS209214), Tfeb (siTFEB#1, Cat# TfebMSS238271; siTFEB#2, Cat# TfebMSS238272), and a negative control (Cat# 12935300), were purchased from Thermo Fisher Scientific. pEGFP-N1-TFEB was purchased from Addgene [Watertown, MA, USA, Addgene plasmid# 38119, gifted by Shawn Ferguson12)]. pAcGFP-C1 vector was purchased from Takara Bio (Shiga, Japan, Cat# CLN632470).
Cell Culture and TransfectionMurine motoneuron-like cells (NSC-34) were purchased from Cosmo Bio Co., Ltd. (Tokyo, Japan) and maintained in Dulbecco’s modified Eagle’s medium (DMEM, Nacalai Tesque, Kyoto, Japan), containing 10% fetal bovine serum (FBS, Valeant, Costa Mesa, CA), as described previously.18) Lipofectamine RNAiMAX and Lipofectamine 2000 were used as transfection reagents, according to the manufacturer’s instructions (Thermo Fisher Scientific). To induce differentiation, one day after seeding into 6-well plates, at 20,000 cells per wells, NSC-34 cells were incubated with DMEM, containing 50 µM all-trans retinoic acid (Sigma-Aldrich, Cat# R2625) and 2% horse serum (Sigma-Aldrich, Cat# H1138), for 3 days, in a humidified atmosphere of 95% air and 5% CO2 at 37°C. The percentage of neurite-bearing cells reached to 57.7 ± 9.3% on the day 3.
Cell Lysis and ImmunoblottingCell lysis and immunoblotting were performed, as previously described.19) Briefly, cells cultured in a 12-well plate were rinsed with ice-cold PBS and lysed in radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich, R0278), containing protease inhibitor cocktail, and phosphatase inhibitor cocktail 2 and 3 (Sigma-Aldrich), and then centrifuged at 12,000 × g, for 20 min at 4°C. The supernatants were collected as cell lysates and boiled with sample buffer solution (FUJIFILM-Wako, Osaka, Japan), for 5 min. Protein extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), in a 5%–20% gradient gel (SuperSep Ace, FUJIFILM-Wako), and transferred to a polyvinylidene difluoride membrane (Immobilon-P, Millipore, Bedford, MA, USA). After blocking with Blocking One-P (Nacalai Tesque), the membranes were incubated with primary antibody, in Can Get Signal Solution-1 (Toyobo, Shiga, Japan), overnight at 4°C, with gentle agitation. After three washes with Tris-buffered saline (TBS), containing 0.05% Tween 20, the membranes were incubated with an HRP-conjugated secondary antibody, in Can Get Signal Solution-2 (Toyobo), for 1 h at room temperature. After three washes with TBS, containing 0.05% Tween 20, the chemiluminescent signal was visualized with ImmunoStar LD (Fujifilm-Wako) and then detected using an Amersham Imager 680 (GE Lifescience, Chicago, IL, USA). The band intensity was analyzed by Amersham Imager 680 Analysis Software (GE Lifescience).
Quantitative RT-PCRQuantitative reverse transcriptase-polymerase chain reaction (RT-PCR) was performed, as described previously.20) In brief, total RNA was purified from NSC-34 cells, grown in a 12-well plate (Corning), using NucleoSpin RNA (Takara Bio), and subjected to reverse transcription to generate a cDNA library, using PrimeScript RT reagent Kit (Takara Bio). Quantitative RT-PCR was performed on a Thermal Cycler Dice Real-Time System III (Takara Bio), using TB Green Premix Ex Taq II (Takara Bio). The primer pairs used in this study were as follows; Gapdh-F, 5’-AACTTTGGCATTGTGGAAGG-3'; Gapdh-R 5’-ACACATTGGGGGTAGGAACA-3'; Tfeb-F, 5’-TCAGAAGCGAGAGCTAACAGAT-3'; Tfeb-R 5’-TGTGATTGTCTTTCTTCTGCCG-3'; Lamp1-F, 5’- CAGCACTCTTTGAGGTGAAAAAC-3'; Lamp1-R 5’-ACGATCTGAGAACCATTCGCA-3'; Ctsd-F, 5’- GCTTCCGGTCTTTGACAACCT-3'; and Ctsd-R, 5’- CACCAAGCATTAGTTCTCCTCC-3'. CT values measured Thermal Cycler Dice Real-Time System software (Takara Bio) by were normalized against Gapdh and expressed as the fold-change in gene expression.
ImmunostainingImmunostaining was performed as previously described, with minor modifications.21) Briefly, cells were grown on 12-mm glass coverslips (No. 1-S, Matsunami Glass Industry, Osaka, Japan), coated with 0.2% gelatin, from porcine skin (Sigma Aldrich, G2500), and fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in PBS-C/M [phosphate-buffered saline (PBS), containing 0.2 mM CaCl2, 2 mM MgCl2], for 10 min at room temperature, quenched with 50 mM NH4Cl, for 10 min, followed by 30 min in blocking buffer [PBS-C/M containing 0.5% bovine serum albumin (BSA), 0.5% saponin, 0.2 mg/ml sodium azide]. The samples were incubated with primary antibodies in PBS-C/M, for 1 h. After three washes with PBS-C/M, cells were stained with secondary antibodies in PBS-C/M, containing 8.1 µM Hoechst 33342 (Thermo Fisher Scientific), for 30 min. For F-actin staining, the samples were stained with Phalloidin-iFluor 594 (Cayman Chemical, Ann Arbor, MI, USA, 1:2,000) together with the secondary antibody. After three washes with PBS-C/M, the coverslips were mounted with ProLong Gold Antifade reagent (Thermo Fisher Scientific). Images were acquired, using either a 40× or 100× objective lens, on a Zeiss LSM700 microscope (Carl Zeiss, Germany). Images were edited using ImageJ (National Institutes of Health) or Adobe Photoshop CC2019 (Adobe), for presentation purposes. To analyze TFEB foci-positive cell ratios, images were analyzed by a third person, in a blinded fashion.
DQ-Red BSA Lysosomal Activity AssayThe DQ-Red BSA (Thermo Fisher Scientific) assay was performed, according to the manufacturer's instructions. Briefly, cells were incubated with 10 µg/ml DQ-Red BSA, for 3 h at 37°C. Hoechst 33342 solution was added, 15 min before recording, to a final concentration of 8.1 µM. For live-cell imaging, the medium was changed to Live Cell Imaging Solution (LCIS, Thermo Fisher Scientific), containing 5.5 mM glucose and 1% FBS, and then images were acquired, using a 10× objective lens on a Lionheart FX Automated Microscope (Bio Tek Instruments, Winooski, VT, USA). The obtained images were analyzed by Gen5 software (Bio Tek Instruments).
Animal ExperimentsSMNΔ7 mice (mSmn-/-, SMN2+/+, SMNΔ7+/+) were purchased from Jackson Laboratory (Bar Harbor, ME, USA, Stock Number 005025) and maintained, as described previously.19) Lumbar spinal cords, from either SMNΔ7 mice or their wild-type (WT) counterparts (mSmn+/+, SMN2+/+, SMNΔ7+/+), were harvested at postnatal day 11 and homogenized, using Physcotron (Microtec Co., Chiba, Japan) in RIPA buffer, containing protease inhibitor cocktail, and phosphatase inhibitor cocktail 2 and 3 (Sigma-Aldrich), then centrifuged, at 12,000 × g, for 20 min at 4°C. The supernatants were examined by immunoblotting, as tissue lysates. All animal manipulations were performed in accordance with the animal care guidelines issued by the National Institutes of Health. All experiments were approved by the Institutional Animal Care and Use Committee of Gifu Pharmaceutical University (Approval No. 2019-078, 2019-190).
Statistical AnalysisAll data are presented as the mean ± standard error of the mean (S.E.M.). Statistical analyses were performed by Student’s t-tests, using SPSS Statistical Software (IBM, Armonk, NY, USA).
NSC-34 cells, a motoneuron-like hybrid cell line, have been used as an in vitro model because of their physiological properties.22) To silence SMN expression in NSC-34 cells, two siRNAs, targeting different regions of Smn, were validated by immunoblotting (Fig. S1A and B). The expression of SMN was significantly decreased, 24 h after transfection, and reduced expression persisted until 72 h after transfection (Fig. 1A and B). Coincidentally, we found that TFEB expression also decreased 48 h and 72 h after transfection (Fig. 1A and C). The mRNA levels of Tfeb also decreased at the 48 h time point (Fig. 1D), suggesting that the transcription of TFEB was suppressed by SMN KD. At steady state, TFEB exists in the cytosol, and activated TFEB is shuttled into the nucleus, to promote lysosomal gene expression.11) In control NSC-34 cells, endogenous TFEB staining appeared as bright cytosolic foci, in addition to signals in the nuclei (Fig. 1E). Consistent with the immunoblotting and quantitative RT-PCR results, TFEB-positive foci disappeared in SMN-depleted cells (Fig. 1E and F). These foci were diminished following the transfection of siRNA targeting TFEB expression (Fig. S2A-C), suggesting that these signals were neither background nor artifacts.

TFEB Expression Levels Decrease in SMN-Depleted NSC-34 Motoneuron-Like Cells
(A-C) Immunoblotting of cell lysates, obtained from NSC-34 cells, transfected with either negative control RNA (- or NC) or siRNA against SMN (siSmn + or SMN KD), for the indicated times. Representative images of the blots are shown in A. The quantitation of SMN (B) and TFEB (C) signals were normalized against β-actin and are shown the fold-change compared with 0 h. (D) The mRNA level of Tfeb, at the 48 h time point, normalized against Gapdh. (E) Immunostaining for TFEB (green), in SMN KD cells. Nuclei are stained with Hoechst 33342 (blue). Arrowheads represent TFEB-positive foci. Bar = 20 µm. (F) The ratio of TFEB foci-positive cells, in either NC or SMN KD. N = 6, mean ± S.E.M. *P < 0.05, **P < 0.005, ***P < 0.001 Student’s t-test vs NC.
All-trans retinoic acids can induce the differentiation of NSC-34 cells.23) We performed immunostaining against the lysosomal marker Lamp1, in differentiated NSC-34 motoneurons (Fig. 2A) because TFEB is important for lysosomal biogenesis and distribution. Differentiated NSC-34 cells displayed long processes, which could be labeled by the neuronal marker β-tubulin III (Fig. 2B–D). The membranes of the neurite branches were highlighted by F-actin staining, and the distal regions of the neurites were enriched with Lamp1-positive granular structures (Fig. 2B, asterisks). Interestingly, these structures disappeared in both SMN KD and TFEB KD cells (Fig. 2C and D). Instead, we found that lysosomes became swollen and enlarged, and these irregularly shaped lysosomes accumulated in the cell bodies (soma) of KD cells (Fig. 2C and D). The shapes of lysosomes have been associated with acidity and degradation.24) Therefore, these results indicate that lysosomal function was disrupted by SMN KD.

SMN and TFEB KD Alter the Distribution and Morphology of Lysosomes in Differentiated NSC-34 Cells
(A) A schematic diagram for the timeline of transfection and induction. NSC-34 cells were differentiated by treatment with all-trans retinoic acid, after transfection with siRNA against either SMN or TFEB or negative control RNA (NC). (B-C) Representative images of immunostaining against Lamp1 (green, top left), F-actin (magenta, top right), β-Tubulin III (cyan, second lane left), and Hoechst 33342 (blue). The boxed regions at the soma (S) and neurites (N) are enlarged in the third and bottom rows, respectively. Asterisks and arrows represent lysosomes at the neurites and soma, respectively. Arrowheads indicate the abnormal structures of lysosomes. Bars = 100 µm.
TFEB regulates lysosome-related gene expression.11) As expected, the mRNA levels of both Lamp1 and cathepsin D (CTSD) were reduced in SMN KD cells (Fig. 3A). Consistently, the protein levels of Lamp1 and CTSD were reduced at the 72 h time point (Fig. 3B–D). Along with lysosomes, TFEB also regulates autophagic gene expression and the induction of autophagy.25) Immunoblotting for LC3 revealed that SMN KD increased autophagic flux (Fig. 3B and E). To investigate the lysosomal activity, we performed the DQ-Red BSA assay. SMN KD cells showed reduced DQ-Red BSA fluorescence intensity compared with that of control cells (Figs. 3F and S3A). Taken together, these results indicated that SMN expression is essential for maintaining lysosome level, its degradation and autophagic activity in motoneurons.

SMN KD Affects Lysosome-Related Gene Expression, Lysosomal Degradation, and Autophagy Flux
(A) The mRNA levels of Lamp1 and Ctsd at the 48 h time point, normalized against Gapdh. (B) Immunoblotting of NSC-34 cell lysates, transfected with either negative RNA (- or NC) or siRNA against SMN (siSmn + or SMN KD), for the indicated time points. (C-E) The quantification of B. The signals for Lamp1 (C) and CTSD (B) were normalized against that for β-actin and are shown as the fold-change relative to the level at 0 h. (E) The ratio of LC3-ii (lower band) vs LC3-i (upper band). (F) The fluorescent intensity of DQ-Red BSA in NSC-34 cells, transfected with negative control or siSmn siRNA, for 72 h, shown as the fold-change vs non-transfected cells. N = 6, mean ± S.E.M.*P < 0.05, ***P < 0.001 Student’s t-test vs NC.
To exclude the possibility that SMN KD directly suppresses the expression of lysosome-related genes, we performed a rescue experiment, in which we transiently expressed exogenous TFEB in SMN-depleted cells (Fig. 4A). Indeed, the exogenous expression of TFEB ameliorated the LC3 ii/i ratio (Fig. 4B and C) and increased the expression of Lamp1 (Fig. 4B and D) but did not affect the expression of SMN, suggesting that TFEB is downstream of SMN. Consequently, the lysosomal and autophagy defects observed in SMN KD cells were likely mediated by decreased TFEB expression.

Exogenous Expression of TFEB Rescues the Depletion SMN in NSC-34 Cells
(A) The experimental design for siRNA and plasmid DNA transfection. After 24 h transfection with either negative control (NC) or SMN (siSmn) siRNA, cells were transfected with either TFEB-GFP or GFP and incubated for an additional 48 h. (B-D) The harvested cell lysates were analyzed by immunoblotting. Representative images are shown in B. The ratio of LC3-ii vs LC3-i and the fold increase in Lamp1 levels, normalized against the β-actin level, are shown in C and D, respectively. N = 4-5, mean ± S.E.M. *P < 0.05, Student’s t-test vs “NC + GFP”. †P < 0.05, Student’s t-test vs “SMN KD + GFP”.
Lysosomes play a key role in the mTOR signaling pathway.14) Axonal translation mTOR activity has been reported to be inhibited by miR-183 in SMN KD primary neurons.26) Consistent with previous reports, SMN KD decreased mTOR phosphorylation (Fig. 5A and B). In addition to mTOR, the phosphorylation of both p70S6K and S6RP was also reduced under the same conditions (Fig. 5C and D). Although SMN KD causes apoptosis in NSC-34 cells,27) defects in the mTOR signaling pathway would also contribute to the loss of motoneurons in SMA.

SMN KD Suppresses mTOR Signaling in NSC-34 Cells
Immunoblotting of cell lysates, obtained from NSC-34 cells, transfected with either negative control (- or NC) or SMN (siSmn + or SMN KD) siRNA, for the indicated times. (A) Representative images of each blot. (B-D) The ratio between the phosphorylated form and the total protein is shown as the fold change relative to the level at 0 h. N = 5, mean ± S.E.M. *P < 0.05, **P < 0.005, Student’s t-test vs NC.
To further investigate motoneurons in the spinal cord, we used SMNΔ7 mice (mSmn-/-, SMN2+/+, SMNΔ7+/+), as an SMA animal model.19,28) The lumbar spinal cords were harvested at postnatal day 11. TFEB and Lamp1 levels were significantly decreased in SMNΔ7 mice (Fig. 6A-C), compared with their counterparts. As well as mTOR, the phosphorylation of S6RP was drastically reduced in SMNΔ7 mice (Fig. 6D–F). Interestingly, in addition to the reduced phosphorylated form, the total level of S6K was decreased in SMNΔ7 mice (Fig. 6 D–F). In summary, SMN plays a crucial role in the expression of lysosome-related genes and mTOR signaling pathways in spinal cords, in vivo.
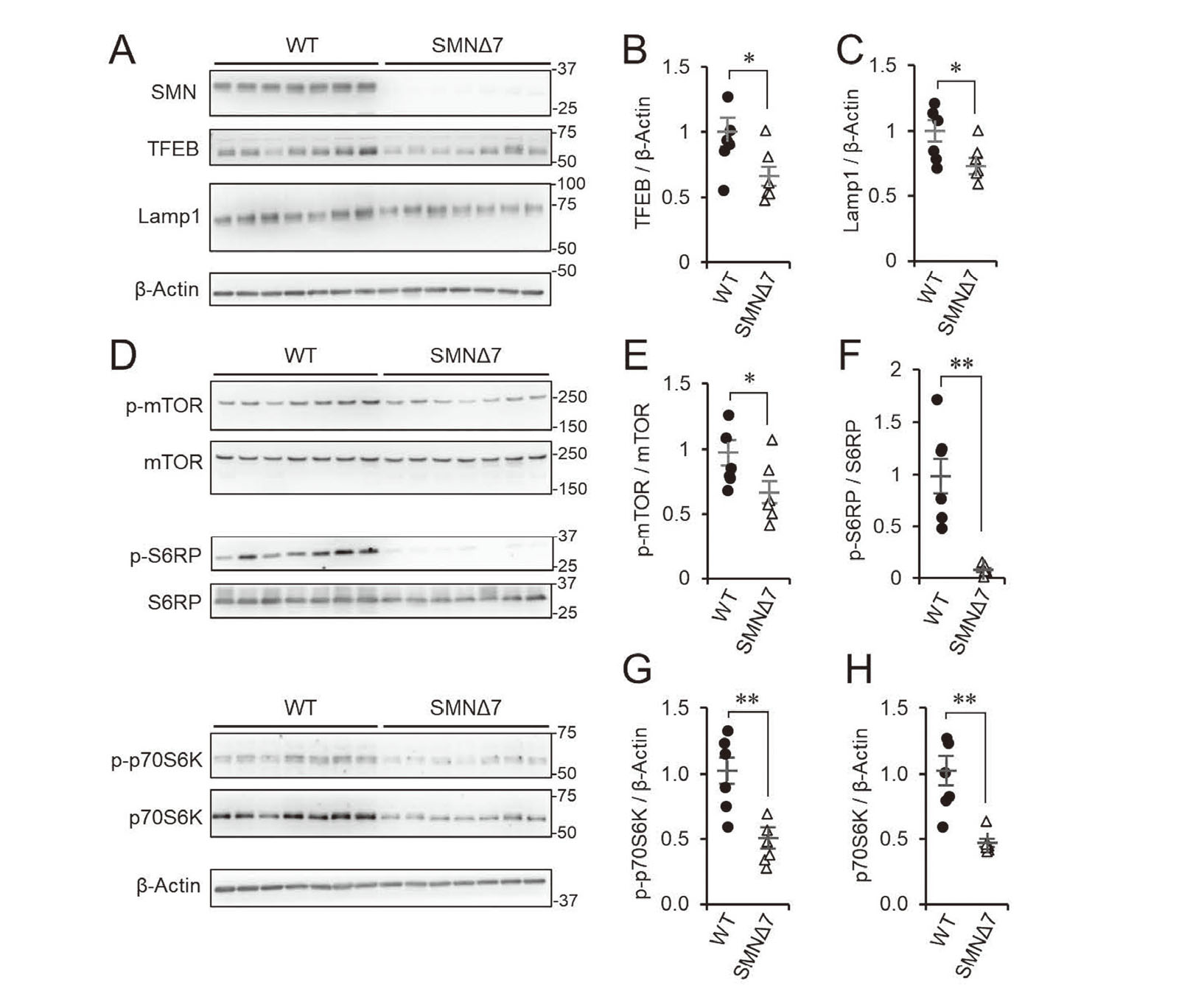
SMA Model Mice Exhibit Defects in the TFEB and mTOR Pathway, in Lumbar Spinal Cords
A and D show immunoblots for lysates obtained from P11 SMNΔ7 (mSmn-/-, SMN2+/+, SMNΔ7+/+) and WT (mSmn+/+, SMN2+/+, SMNΔ7+/+) mice. The quantifications of A and D are shown in B–C and E-H, respectively. N = 7, mean ± S.E.M. *P < 0.05, **P < 0.005, Student’s t-test vs WT.
In the present study, we investigated the functional interaction between SMN and TFEB, in motoneurons. SMN KD reduced TFEB expression, at the transcriptional level (Fig. 1). In addition to a reduction in lysosome-related gene expression, the lysosome dysfunction and increased autophagy influx were observed in SMN-depleted cells (Fig. 3). Consistent with the results obtained from NSC-34 cells, defects in the TFEB and mTOR pathway were detected in the lumbar spinal cords of SMA model mice (Fig. 6). The decline in lysosomes and autophagy was rescued by exogenously expressed TFEB (Fig. 4). Therefore, the reduced intracellular degradation caused by SMN depletion is mediated by the loss of TFEB expression. Previous report showed that caspase-3 activity significantly increased at 72 h after post transfection of SMN siRNA in NSC-34 cells 27), whereas we found that TFEB level was already declined at an earlier time point 48 h, suggesting that TFEB down regulation can occur prior to apoptosis. Further study remains necessary to clarify the molecular mechanisms involved in the SMN regulation of TFEB expression and the correlation between TFEB mediated pathway and motoneuron loss.
Intriguingly, we found that the distribution and morphology of lysosomes were altered in SMN-depleted cells (Fig. 2). The endocytic pathway and the degradation of endocytosed components are essential for neurotransmitter secretion and the maintenance of synaptic plasticity.29) Axonal lysosomes and macroautophagy have been reported to play important roles in axonal development.30,31) Lysosomal activity is also involved in trans-synaptic communications at the NMJ.32) Motoneurons have long axons; thus, the delivery of lysosomes might be crucial for their survival. TFEB has been associated with the intracellular distribution of lysosomes, by regulating their delivery.33) Understanding the molecular mechanism associated with the axonal delivery of lysosomes might be a key to understanding the sensitivity of motoneurons to the loss of SMN expression.
On the membrane of lysosomes, mTORC1 works as a sensor for metabolic status, regulating protein and lipid synthesis for cell survival.14) The local axonal translation in motoneurons is regulated by mTOR signaling.15,26) Consistent with a previous report, SMN depletion in NSC-34 cells also resulted in decreased mTOR phosphorylation and its downstream effectors, p70S6K and S6RP (Fig. 5), suggesting that the deactivation of mTOR might be associated with motoneuron loss in SMA. In addition, axon outgrowth has been reported to be influenced by the PI3K/mTOR pathway,12) indicating that mTOR dysfunction in SMA might cause the deterioration of the NMJ. mTOR regulates the activation and nuclear translocation of TFEB.12,13) In the present study, whether the loss of TFEB following SMN KD occurs in an mTOR signaling-dependent manner was not determined. Further investigations are necessary to reveal their causative relationships and roles in motoneurons and SMA pathophysiology.
Several treatments have been approved for SMA. Nusinerisen (SpinrazaTM, Biogen) is an antisense oligo that promotes the production of mature SMN2, including exon 7, by masking the regulatory sequences required for exon 7 splicing, which, consequently, increases the levels of functional SMN protein.34) Onasemnogene Abeparvovec-xioi (Zolgensma™, Novartis), also known as AVXS-101, is an Adeno-associated virus 9 that codes the SMN protein, for the replacement of the target gene.35) Although these treatments remediate the defects associated with SMN deficiency, by enhancing SMN levels in the long term, developing new treatments that prevent or reduce neuronal loss would be beneficial for SMA patients. SMA patient-derived pluripotent stem cell-derived cells are useful tools for chemical screening, and some antioxidants have been identified that display protective effects against motoneuron loss.36,37) The aberrant deposition of 4-hydroxy-2-nonenal in spinal motoneurons of SMA patients has been reported,38) and differentiated NSC-34 cells are sensitive to ferroptosis,39) an iron-dependent programmed cell death pathway,40) suggesting that motoneurons are relatively sensitive to oxidative stress. Lysosomes and autophagy are responsible for the clearance of lipid peroxides.41) Motoneurons may display characteristic features that rely on these degradative organelles to reduce oxidative stress, suggesting that chemicals capable of decreasing oxidative stress or increasing the degradation of oxidative-stress-associated molecules may represent drug candidates for SMA.
In conclusion, we demonstrated that SMN regulates the gene expression of TFEB, and consequently, the functions of lysosomes and autophagy. These findings reveal the novel roles of SMN and TFEB during lysosome homeostasis and motoneuron survival.
This work was Grant‐in‐Aid for Research Activity Start-up from the Japan Society for Promotion of Science [No. 19K23707 to WO].
Conflict of interestThe authors declare no conflict of interest.