2020 年 3 巻 4 号 p. 138-141
2020 年 3 巻 4 号 p. 138-141
Polymethoxyflavones (PMFs) are flavone compounds that contain more than two methoxyl groups and are almost exclusively found in citrus peel. In this study, we examined and compared the effects of the PMFs nobiletin, heptamethoxyflavone and natsudaidain on antigen-specific T cell activation in vitro and in vivo. The three PMFs suppressed proliferation responses in an in vitro study. The PMFs also suppressed IFN-γ, IL-2 and IL-10 production from splenocytes in a dose-dependent manner. Interestingly, nobiletin and heptamethoxyflavone, but not natsudaidain, enhanced IL-4 production from splenocytes. In an in vivo model, antigen-specific T cell proliferation responses were reduced in mice treated with nobiletin, heptamethoxyflavone and natsudaidain compared with responses in control mice. The results suggest that citrus PMFs suppress T cell activation and that nobiletin and heptamethoxyflavone enhance IL-4 production.
The immune system consists of innate immunity and acquired immunity. The innate system is nonspecific and is the primal part of the immune defense. These are backed up by chemical and microbiologic barriers within mucous membrans, which may limit a pathogen’s proliferation and spread within the host. The acquired immune response takes over if the innate response cannot clear an infection in a short time. Induction of T cell and B cell responses play a role in acquired immunity and can induce strong antigen (Ag)-specific responses and then acquired immunological memory. T cells activated by Ag-presenting cells produce cytokines and regulate systemic host immune responses.1)
Citrus flavonoids are composed of three major subgroups: flavanones, flavone glycosides and polymethoxyflavones (PMFs). PMFs are flavone compounds with several methoxyl groups that exhibit a wide range of physiological and pharmacological actions. Nobiletin (5,6,7,8,3’,4’-hexamethoxyflavone, NOB) is one of the most extensively studied PMFs. NOB has been shown to be have various beneficial effects including anti-cancer,2) anti-inflammation,3) anti-oxidation,4) anti-insulin resistance,5) anti-osteoclastogenesis,6) cardiovascular protection7) and neuroprotection effects.8) Natsudaidain (3,6,7,8,3’,4’-hexamethoxyflavone) (NTD) is a PMF that has 6 methoxy groups similar to those of NOB, but the position of the methoxy group is different from that in NOB. Heptamethoxyflavone (3,5,6,7,8,3’,4’-hexamethoxyflavone) (HMF) has 7 methoxy groups. In this study, we examined and compared the effects of the PMFs NOB, HMF and NTD on antigen-specific T cell activation in vitro and in vivo.
NOB, NTD and HMF were provided by Ushio-Chemix Co. (Shizuoka, Japan). Ovalubumin (OVA)323-339 peptide was synthesized by Sigma-Aldrich (MO, USA) and the purity was over 97%. 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was obtained from Tokyo Chemical Ind. Co. Ltd. (Tokyo, Japan).
MiceBALB/c mice (Japan SLC, Shizuoka, Japan) and DO11.10 mice on a BALB/c background (The Jackson Laboratory, ME, USA) were maintained under specific pathogen-free conditions with a 12-h light:dark cycle at 25 ± 2°C and 55 ± 10% relative humidity. The mice were given free access to water and food throughout the experiment. All studies were performed in accordance with the ethical guidelines for animal experimentation by the Institution of Biomedical Sciences, The University of Tokushima, Japan and were approved by the institution review board of the animal ethics committee.
Proliferation AssaySplenocytes (5 x 105 cells/well) from DO.11.10 mice were treated with NOB, NTD or HMF for 24 h in 96-well flat-bottom plate and then further cultured with 5 µg/mL OVA323-339 peptide for 48 h at 37°C in a total volume 100 μL. Twenty μL of MTT solution was added to the culture 4 h before the end of culture. Fifty μL of 10% SDS solution was added to the well and incubated overnight at 37°C. Absorbance at 550/630 nm was measured using a microplate reader.
Cytokine ProductionSplenocytes (2.5×106 cells/well) from DO11.10 mice were pretreated with NOB, NTD or HMF in a 48-well flat-bottom plate at 37°C under 5% CO2 for 24 h and then the cells were stimulated with 5 µg/mL OVA OVA323-339 peptide for 48 h. After the culture, culture supernatants were collected and stored at -30°C until used. Interferon (IFN)-γ, interleukin (IL)-2, IL-4 and IL-10 in the supernatants were quantified using mouse IFN-γ, IL-2, IL-4 and IL-10 (Biolegend, San Diego, CA, USA) enzyme-linked immunosorbent assay (ELISA) kits according to the instructions of the manufacturers.
Immunization and Ag-Specific T Cell ResponseMice were immunized intraperitoneally with 500 μL of OVA solution containing 10 µg of OVA (Sigma Chemical Co., MO, USA) absorbed in 2 mg of aluminium hydroxide gel adjuvant (Sigma-Aldrich Co., MO, USA) on days 7 and 21 after the start of administration of PMFs. OVA-immunized mice were given 100 mg/kg body weight PMFs orally from before 1 week to after 4 weeks of immunization. Two weeks after the final immunization, spleens were collected and single cells were prepared from the spleens. Splenocytes (5×105 cells/well) were cultured with 400 μg/mL OVA in a 96-well flat-bottom plate at 37°C under 5% CO2 for 72 h. For the last 8 h of culture, 37 KBq of [3H]thymidine deoxyribose (TdR) was added to the wells, and the amount of [3H]TdR incorporated was measured by a scintillation counter (Aloka, Tokyo, Japan).
Statistical AnalysisData shown are representative of at least two or three experiments. Significance was analyzed using one-way ANOVA followed by Scheffe’s post hoc test for multiple comparisons. Data are expressed as means ± standard error (SEM). Differences were considered significant at P < 0.05.
We focused on the effects of PMFs on T cell proliferation response. The PMFs we used were NOB, NTD and HMF, which contain 6 or 7 methoxy groups and have similar structures (Fig. 1 A). The DO11.10 mice used in this study are transgenic mice that carry the OVA-specific T cell receptor gene.9) We first examined the effects of PMFs on Ag-specific T cell activation using DO11.10 mouse splenocytes. PMFs significantly suppressed T cell activation in a dose-dependent manner. The addition of 25 μM of each PMF reduced the value to the base line level. The suppressive activities of NOB was stronger than the suppressive activities of NTD and HMF (Fig. 1 B).
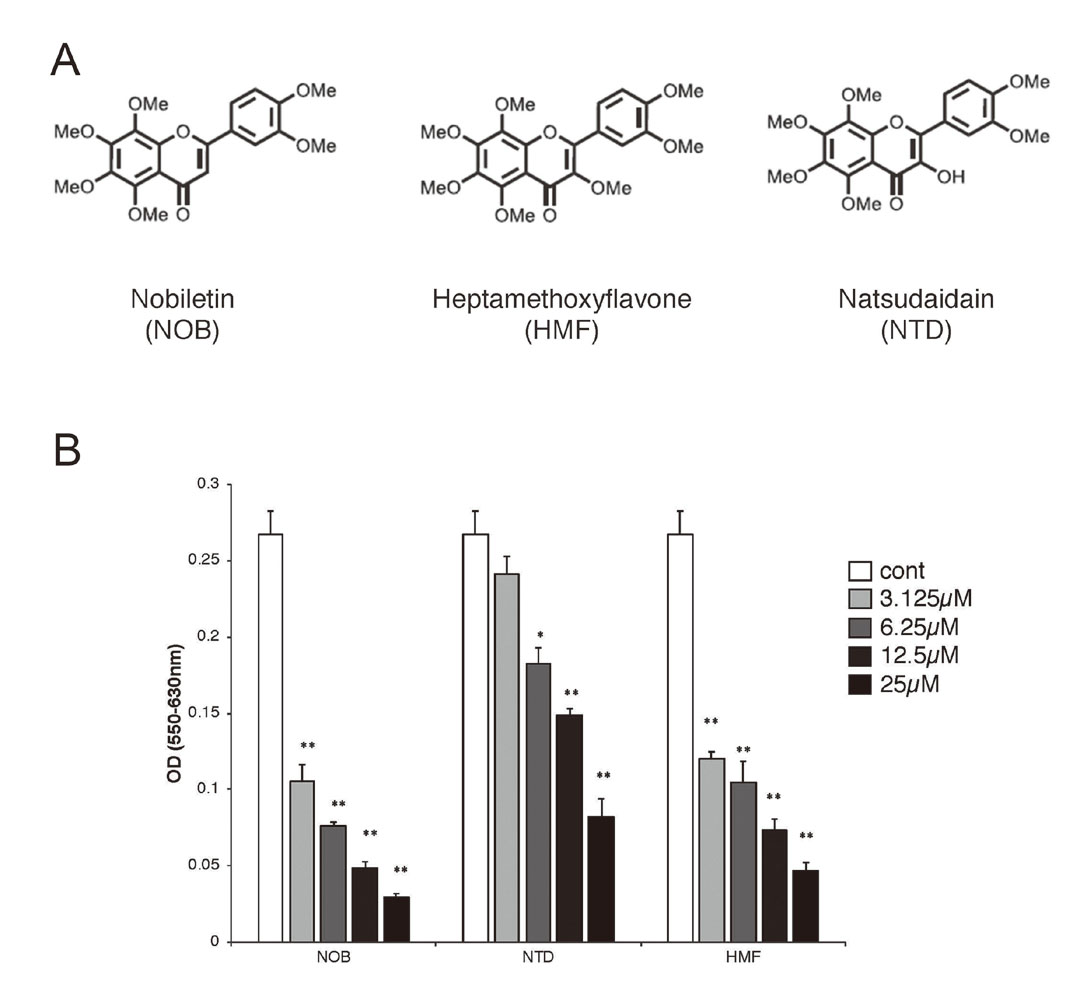
PMFs Suppress Ag-Specific T Cell Responses
(A) Chemical structures of NOB, HMF and NTD are shown. (B) Splenocytes from DO11.10 mice were pretreated with each PMF for 24 h and then stimulated with OVA323-339 peptide for 48 h. Cell growth was determined by the MTT assay. Value of proliferation response in splenocytes not stimulated with OVA was 0.042±0.005. Data are shown as means±SEM. * P < 0.05 vs the value for stimulation with OVA peptide alone (white bar). ** P < 0.01 vs the value for stimulation with OVA peptide alone (white bar).
In addition to the proliferation response, we observed cytokine production from T cells. Significant suppression of INF-γ production was observed at relatively small concentrations of PMFs. The PMFs also suppressed IL-2 and IL-10 production in a dose-dependent manner. A drastic change was observed in IL-4 production. NOB and HMF enhanced Ag-specific IL-4 production in a dose-dependent manner, whereas NTD did not affect IL-4 production (Fig. 2).

Effects of PMFs on Production of Ag-Specific Cytokines
Splenocytes from DO11.10 mice were pretreated with each PMF for 24 h and then stimulated with OVA323-339 peptide for 48 h. Concentrations of IFN-γ, IL-2, IL-4 and IL-10 were determined by ELISA. Value of IFN-γ, IL-2, IL-4 and IL-10 concentration in splenocytes not stimulated with OVA was < 500 pg/mL, < 250 pg/mL. < 5 pg/mL and < 10 pg/mL, respectively. Data are shown as means±SEM. * P < 0.05 vs the value for stimulation with OVA peptide alone (white bar). ** P < 0.01 vs the value for stimulation with OVA peptide alone (white bar).
We also examined Ag-specific T cell induction in OVA-immunized mice. BALB/c mice were treated with PMFs and immunized with OVA twice. Splenocytes from the mice that received the treatment were stimulated with OVA in vitro and proliferative responses were determined. Ag-specific T cell response in mice treated with PMFs was weak compared to that in control mice (Fig. 3).

PMFs Suppress Ag-Specific T Cell Induction In Vivo
BALB/c mice were treated with PMFs and immunized with OVA twice. Splenocytes from OVA-immunized mice were stimulated with 200 mg/mL OVA for 72 hr. Proliferative response was determined by incorporation of 3H[TdR]. Value of 3H[TdR] incorporation in splenocytes not treated with OVA was 838±59 cpm. Data are shown as means±SEM. * P < 0.05 vs control mice. ** P < 0.01 vs control mice.
It has been shown that PMFs, especially NOB, have various physiological functions. However, there are few comparative study of PMFs. In this study, we compared the effects of three PMFs on Ag-specific T cell response in vitro and in vivo. The main findings of this study are as follows: 1) PMFs suppressed Ag-specific T cell response in vitro and in vivo and 2) PMFs suppressed Ag-specific IFN-γ, IL-2 and IL-10 production, whereas NOB and HMF enhanced Ag-specific IL-4 production.
The bioactivities of a compound depend on its structure and its metabolism. There have been some studies in which the effects of biological actions of NOB and other PMFs were compared. Inhibitory activity of histamine and TNF-α releases from basophil-like RBL-2H3 cells by NOB has been shown to be same as that by NTD.10) In another study by Young et al., psoriasis-like skin lesions were induced by 12-O-terradecanoylphorbol-13-acetate in mice, and the preventive effects of NTD and HMF on psoriasis-like skin lesions were evaluated.11) They found that levels of cytokines, including IL-1β, IL-4, IL-6, IFN-γ and TNF-α, were reduced after NOB and HMF treatment. Moreover, HMF showed strong activity in the prevention of psoriasis than did NOB. Although the functions of NOB have been more extensively studied than the functions of other PMFs, there is a possibility that PMFs have analogous functions in inflammatory responses.
Unexpectantly, it was found that NOB and HMF enhanced IL-4 production from T cells, while they suppressed both proliferation response and cytokine productions (Fig. 2). It has been shown that HMF inhibits the proliferation of splenocytes stimulated with anti-CD3/CD28 antibody.12) The results of that study are consistent with our results that showing HMF inhibits Ag-specific T cell activation (Fig. 1). Administration of HMF at a dose of 50 mg/kg weight in mice reduced production of IL-4 from splenocytes stimulated with anti-CD3/CD28 antibody.13) In a previous study, we found that treatment with 20 mg/kg NOB enhanced Ag-specific IL-4 production.14) In this study, we used PMFs at doses of 100 mg/kg and there was no significant difference in IL-4 production from splenocytes between the control and experimental groups (data not shown). Since the results of in vitro study (Fig. 2) showed that enhancement of IL-4 production is dependent on the dose of PMFs, the concentration of a PMF is one of the key factors for IL-4 production from T cells.
In autoimmune animal model of experimental autoimmune encephalomyelitis, IL-17 and IFN-γ from T helper 17 cells have been shown to associated with clinical outcomes.15) We found that PMFs suppressed Ag-specific IFN-γ production in vitro. Furthermore, IL-4 production and the subsequent generation of type 2 T helper responses has been show to important for development of experimental autoimmune encephalomyelitis.16) Elucidation of the positive and negative effects of PMFs on cytokine production might lead to the development of treatment for immune diseases.
This study was supported, in part, by JPSS KAKENHI Grant Numbers JP19K1176800 (T.S.). NOB, NTD and HMF were kindly provided by Ushio-Chemix Co.
Conflict of interestThe authors declare no conflict of interest.