2020 年 3 巻 4 号 p. 142-145
2020 年 3 巻 4 号 p. 142-145
Exposure to cadmium, an environmental pollutant, has been associated with adverse health effects such as atherosclerosis. Nucleolin is a multifunctional protein present in the nucleus, cytoplasm, and cell surface and involved in the angiogenesis and repair of the vascular endothelium following injury. Nucleolin dysfunction has been suggested to cause the development of vascular lesions. In the present study, we assessed the effect of cadmium on nucleolin expression in cultured bovine aortic vascular endothelial cells. After incubation with cadmium (0–5 µM), cellular NCL (nucleolin) mRNA levels and nucleolin protein levels were determined by real-time RT-PCR and western blotting, respectively. Cadmium did not alter nucleolin levels in vascular endothelial cells. We then evaluated whether nucleolin modulates endothelial cadmium-induced cytotoxicity by lactate dehydrogenase leakage. In nucleolin-knockdown vascular endothelial cells, cadmium-induced cytotoxicity was significantly higher than in control cells. Furthermore, nucleolin knockdown did not decrease the mRNA levels of metallothionein 1A and 2A, suggesting that nucleolin protects vascular endothelial cells against cadmium-induced cytotoxicity via metallothionein-independent pathways.
Cadmium is an environmental pollutant that can exert multiple adverse effects1–3) and its most common routes of exposure are drinking water, food, and smoking.4–7) Previous reports have shown that the vascular endothelium is an important target of cadmium cytotoxicity.8–10) Because blood vessels are ubiquitous in the body, injury or functional damage to vascular endothelial cells may influence cadmium cytotoxicity in the parenchymal cells of target organs. Cadmium has also been proposed as a factor in atherosclerosis progression and the underlying mechanisms may involve oxidative stress, inflammation, up-regulation of adhesion molecules, endothelial dysfunction, and altered proteoglycan/glycosaminoglycan synthesis.11) We previously found that cadmium is cytotoxic and destroys the vascular endothelial cell monolayer.12) In addition, we showed that cadmium decreases fibrinolytic activity and alters proteoglycan synthesis in vascular endothelial cells.13,14) These observations indicate that identifying the mechanism of cadmium-induced cytotoxicity and defense mechanisms against the heavy metal damage to vascular endothelial cells may provide information to prevent the progression of cadmium-induced conditions such as atherosclerosis.
Nucleolin is a major nucleolar protein involved in ribosome biogenesis and maturation in eukaryotic cells.15,16) Nucleolin is a multifunctional protein present not only in the nucleus but also in the cytoplasm and on the cell surface.17–19) The role of cell-surface nucleolin in angiogenesis has been studied extensively and the protein has been identified as a marker of angiogenic blood vessels.20,21) Nucleolin has also been reported to be involved in the induction of vascular endothelial growth factor in vascular endothelial cells.22) Since cadmium impairs the endothelial cell monolayer by causing cell detachment and inhibiting cell proliferation,12) we hypothesized that nucleolin may modulate cadmium cytotoxicity in vascular endothelial cells. In the present study, we first investigated the effect of cadmium on nucleolin expression in vascular endothelial cells and then studied the effect of nucleolin on cadmium-induced endothelial injury.
Bovine aortic endothelial cells (Cell Applications, San Diego, CA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 10% heat-inactivated fetal bovine serum (Fujifilm Wako Pure Chemical Industries, Ltd., Osaka, Japan) in a humidified 5% CO2 atmosphere at 37°C until confluence. The medium was removed and the cells were washed twice with serum-free DMEM, then exposed to cadmium chloride (Fujifilm Wako Pure Chemical Industries, Ltd.) at concentrations of 1, 2, 5, or 10 µM in serum-free DMEM for 3, 6, 24, or 48 h.
Real-Time RT-PCRTotal RNA extraction from cultured cells and subsequent real-time RT-PCR were performed as described previously.23) Briefly, after incubation, the culture medium was removed and the cell layer was washed twice with cold calcium- and magnesium-free phosphate-buffered saline (CMF-PBS, Nissui Pharmaceutical Co., Ltd.) and cold ISOGEN II reagent (Nippon Gene, Tokyo, Japan) was added to each culture well. Cells were collected by scraping and homogenized by pipetting. RNA quality was ensured by spectrophotometric analysis (optical density 260/280 nm) using a NanoDrop Lite spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Reverse transcription was performed using ReverTra Ace qPCR RT Master Mix (Toyobo, Osaka, Japan) and a GeneAmp 9700 PCR system (Thermo Fisher Scientific). Real-time PCR was performed using THUNDERBIRD SYBR qPCR Mix (Toyobo) with 0.5 µM of primers and a LightCycler 96 (Roche, Basel, Switzerland). The PCR primers (Supplementary Table 1) were purchased from Star Oligo Rikaken (Aichi, Japan). The fold-change for each gene was assessed after normalization of the intensity value to that of β-actin (ACTB).
Western BlottingWestern blot analysis was performed as described previously with small modifications.23) Confluent cultures of vascular endothelial cells were treated with cadmium chloride (1, 2, or 5 µM) in serum-free DMEM for 24 h. After incubation, the cell layers were washed twice with cold CMF-PBS and lysed with RIPA buffer (1 mM Tris-HCl [pH 7.4], 1% NP-40, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 150 mM sodium chloride, 1 mM EDTA, 1 μg/mL leupeptin, 1 μg/mL pepstatin, and 1 mM PMSF). After collection, the solution was centrifuged at 15,000 × g at 4°C for 5 min. The supernatant was collected and the protein concentration was determined using a detergent-compatible protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to an Immobilon-P membrane (Merck KGaA, Darmstadt, Germany), and visualized using primary antibodies against nucleolin (Santa Cruz Biotechnology, Dallas, TX, USA) and β-actin (Abcam, Cambridge, UK) and a secondary antibody, anti-mouse IgG-horseradish peroxidase antibody (GE Healthcare, Little Chalfont, UK).
siRNA TransfectionNegative control siRNA and nucleolin siRNA (GGAAGUUUGGCUAUGUGGAdTdT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Vascular endothelial cells were transfected with siRNAs using lipofectamine RNAiMAX transfection reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. Briefly, a double-strand siRNA solution (5 pmol: final siRNA volume used per well) was added to RNAiMAX transfection reagent and incubated for 10 min at room temperature to allow siRNA/RNAiMAX complex formation. After incubation, the siRNA/RNAiMAX complex was added to a suspension of vascular endothelial cells (2.5 × 105 cells/mL) and seeded in 400-µL aliquots per well in 24-well culture plates (5 × 104 cells/cm2). After 24 h of incubation, vascular endothelial cells were treated with cadmium chloride (5 µM) in serum-free DMEM for 48 h.
Morphological Appearance and Cytotoxicity AssaysMorphological appearance and cytotoxicity assays were performed as described previously.24) After cadmium treatment, the conditioned medium was harvested and an aliquot was used to measure lactate dehydrogenase (LDH) activity, an indicator of cytotoxicity, using a CytoTox 96 non-radioactive cytotoxicity assay kit (Promega, Madison, WI, USA). Since LDH leakage into the medium is consistent with morphological cell damage,25,26) the activity was not normalized by total LDH activity. For morphological observations, the cell layer was washed with CMF-PBS and then fixed with methanol and stained with Giemsa solution (Merck KGaA). Bright-field imaging was performed with a DMi1 inverted microscope (Leica Microsystems, Wetzlar, Germany). We reported that there is a strong correlation between the morphological appearance and LDH leakage in cultured bovine aortic endothelial cells after exposure to cadmium.12)
Statistical AnalysisAll statistical analyses were performed in Excel (Microsoft, Redmond, WA, USA) with the Statcel3 add-in (OMS, Tokyo, Japan). Data are expressed as means ± SD. The statistical significance of the data was determined using one-way ANOVA with a post-hoc Bonferroni-Dunn test or Student’s t-test as appropriate. Differences between groups were considered significant at p<0.05.
Cadmium did not influence nucleolin expression at any time point (Fig. 1A). In addition, nucleolin protein levels in vascular endothelial cells did not change in response to cadmium treatment (Fig. 1B). These findings indicate that cadmium does not affect nucleolin expression in vascular endothelial cells.

Effect of Cadmium on Nucleolin (NCL) Expression in Vascular Endothelial Cells
Confluent cultures of bovine aortic endothelial cells were incubated for 3, 6, or 24 h with 1, 2, or 5 µM cadmium. A. NCL mRNA levels, determined by real-time RT-PCR. Data are represented as means ± SD (n=3). B. NCL protein levels in cells treated with cadmium for 24 h were analyzed by western blot.
We investigated the cytotoxic effect of cadmium on vascular endothelial cells by morphological observation and LDH leakage. Treatment of endothelial cell monolayers with cadmium resulted in de-endothelialized areas that were not observed in the controls (Fig. 2A). Cadmium caused a significant increase in LDH leakage into the conditioned medium that was concentration-dependent (Fig. 2B). This indicates that cadmium is cytotoxic to vascular endothelial cells under these experimental conditions. siRNA-mediated knockdown of nucleolin expression (the reduction of NCL mRNA and nucleolin protein was 95% and 37%, respectively, as shown in Fig. 2C) further increased LDH leakage (Fig. 2D), suggesting that nucleolin may protect against cadmium cytotoxicity in vascular endothelial cells.
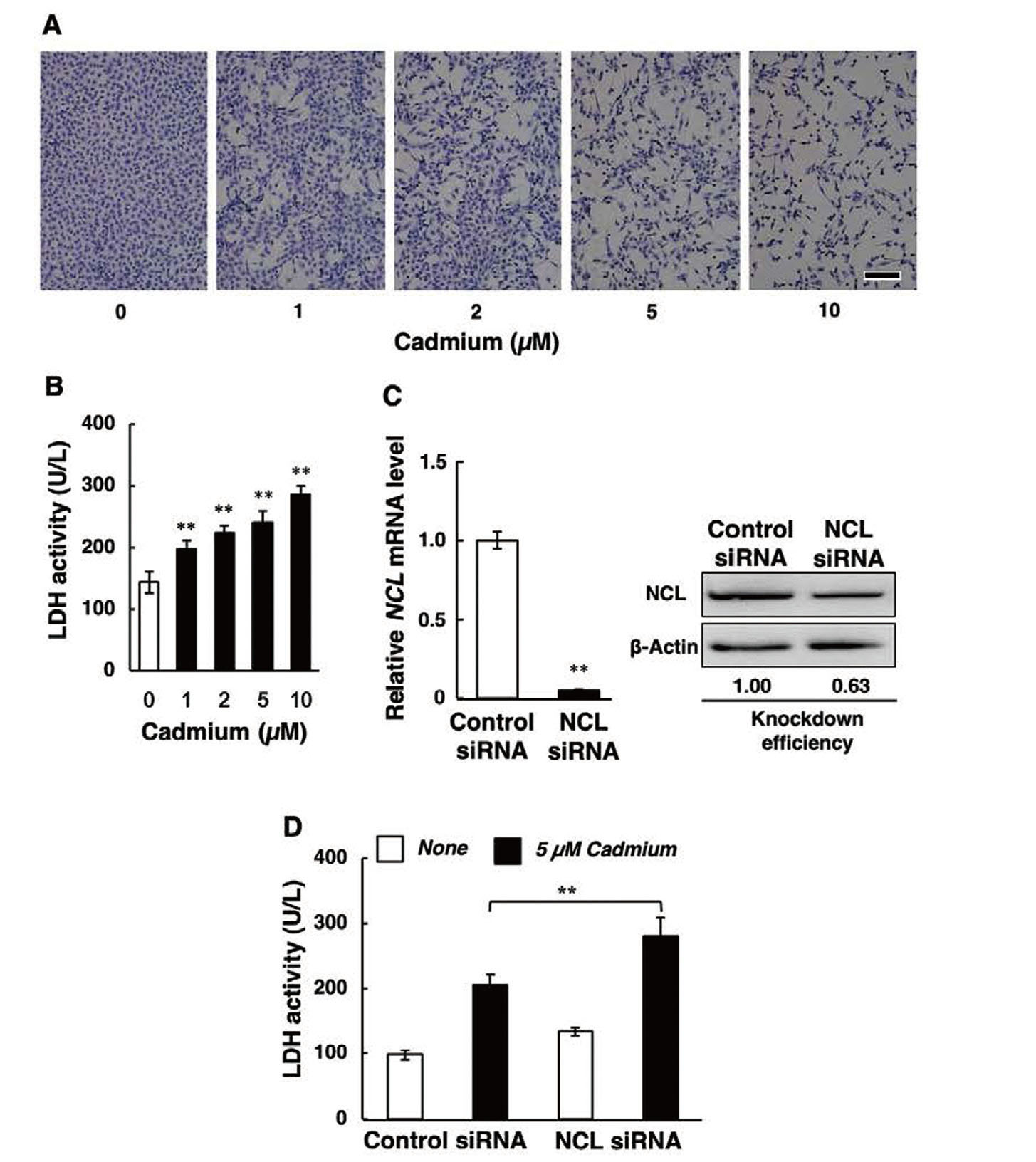
Nucleolin (NCL) Knockdown Enhances Cadmium Cytotoxicity in Vascular Endothelial Cells
A. Representative images of vascular endothelial cells treated with cadmium for 48 h. Scale bar: 200 µm. B. Lactate dehydrogenase (LDH) activity as a marker of cytotoxicity following a 48-h exposure to cadmium. Data are represented as means ± SD (n=4). **p<0.01 compared with levels in controls. C. Knockdown efficiency of NCL in vascular endothelial cells following siRNA treatment. Expression levels of NCL mRNA (left) and NCL protein (right) in controls and in cells treated with NCL siRNA for 24 h are shown. Values are means ± SD (n=3). **p<0.01 compared with levels in controls. D. NCL knockdown vascular endothelial cells were treated with 5 µM cadmium for 48 h. Cytotoxic effects of cadmium were determined by LDH leakage. Data are represented as means ± SD (n=4). **p<0.01 compared with levels in controls.
Metallothionein is cytoprotective against cadmium toxicity.27,28) We previously showed that cadmium induces metallothionein synthesis in bovine aortic vascular endothelial cells,29) and hypothesized that nucleolin protects vascular endothelial cells against cadmium cytotoxicity by inducing metallothionein. However, siRNA-mediated nucleolin knockdown did not alter the mRNA levels of MT1A and MT2A (Fig. 3). Cadmium markedly increased both MT1A and MT2A mRNA levels, which were not lower in nucleolin knockdown cells than in control cells, indicating that protection against cadmium cytotoxicity does not occur via metallothionein induction. In bovine tissues, there are three types of MT genes, namely, MT1A, MT1E, and MT2A. In this study, we focused on MT1A and MT2A (sub)isoforms as MT1 and MT2 genes, respectively, because induction of MT1A gene expression by cadmium was markedly higher than that of MT1E (data not shown). NCL mRNA levels were also unaffected by cadmium treatment (Supplementary Fig. 1). We previously showed that cadmium induces p53-dependent apoptotic cell death.30,31) Nucleolin overexpression has been reported to suppress p53 translation and induction, while the down-regulation of nucleolin was shown to promote p53 expression.32,33) Therefore, it is possible that suppression of p53 expression by nucleolin may modulate cadmium cytotoxicity. We are currently investigating p53-dependent apoptosis as the putative mechanism underlying nucleolin-mediated protection against cadmium cytotoxicity.
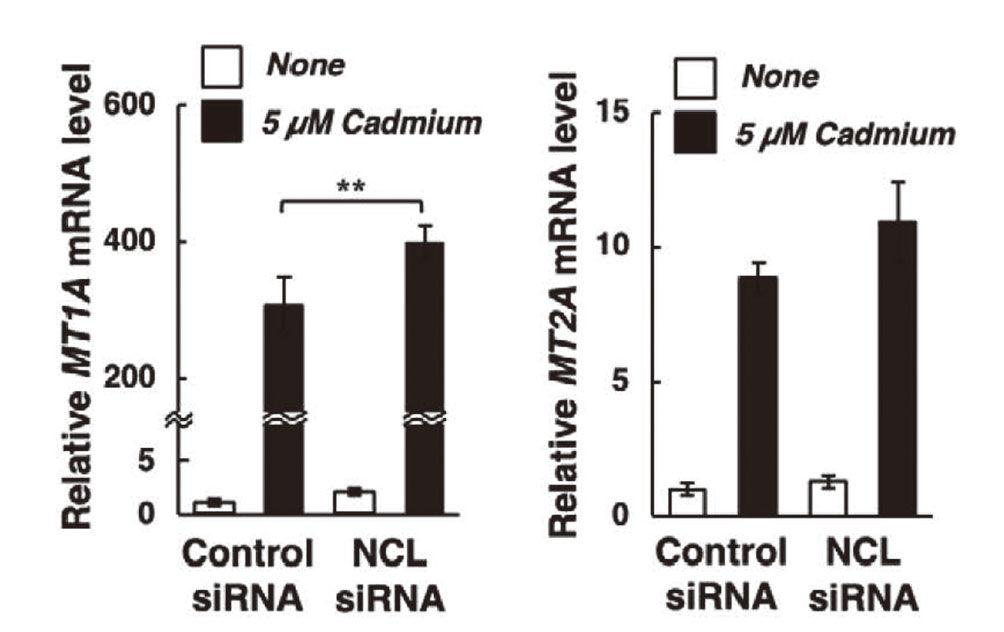
Effect of Nucleolin (NCL) Knockdown on MT1A and MT2A mRNA Expression in Vascular Endothelial Cells Treated with Cadmium
NCL knockdown vascular endothelial cells were treated with cadmium for 24 h. The expression levels of MT1A mRNA (left) and MT2A mRNA (right) were determined by real-time RT-PCR. Values are means ± SD (n=3). **p<0.01 compared with levels in controls.
In conclusion, our findings indicate that cadmium does not influence nucleolin expression but that nucleolin may protect against cadmium cytotoxicity in vascular endothelial cells via metallothionein-independent pathways.
This work was supported by a Grant-in-Aid for Scientific Research (C) JP 16K08350 (to Y.F.) and a Grant-in-Aid for Scientific Research (C) JP 17K08391 (to T.T.) from the Japan Society for the Promotion of Science (JSPS). We thank Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Conflict of interestThe authors declare no conflict of interest.