2021 年 4 巻 1 号 p. 22-26
2021 年 4 巻 1 号 p. 22-26
Cetuximab (Cmab) is known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hypocalcemia. However, little is known about differences in these electrolyte levels between hypomagnesemia and non-hypomagnesemia group in patients receiving Cmab. Therefore, the aim of this study was to clarify the relationship between these serum electrolyte levels in patients undergoing Cmab therapy. A retrospective study was conducted to investigate patients for advanced or recurrent colorectal cancer and head and neck cancer, treated with a regimen composed of Cmab from 2012 to 2020 at the Kindai University Nara Hospital. A total of 113 patients were identified from the medical records, and 24 patients who met the inclusion criteria were enrolled in this study. In the non-hypomagnesemia group, significant positively correlations were observed between calcium and potassium (p = 0.018), between potassium and magnesium (p = 0.008), and between magnesium and calcium (p = 0.038). Simultaneously, in the hypomagnesemia group, no statistically significant correlations were recorded between these electrolytes. The incidences of hypokalemia, hypomagnesemia, and hypocalcemia were 25.0% (6/24), 29.2% (7/24), and 25.0% (6/24), respectively. Additionally, the onset of hypokalemia was significantly associated with the onset of hypocalcemia (p = 0.009). These data suggest that it is important to monitor these electrolyte levels, especially in patients who received Cmab with combination therapy.
Cetuximab (Cmab) is a monoclonal antibody that targets the epidermal growth factor (EGFR), binds EGFR with high affinity, and inhibits tyrosine kinase activation.1) In Japan, Cmab is used for the treatment of unresectable advanced or recurrent colorectal cancer, and head and neck cancer. Reportedly, characteristic adverse effects associated with Cmab therapy include electrolyte abnormalities,2-4) such as hypomagnesemia, hypokalemia, and hypocalcemia, which differ from adverse effects related to other anticancer drugs, including gastrointestinal toxicity and myelosuppression. Electrolyte abnormalities are diseases that cause various symptoms such as hypomagnesemia include tetany, seizures and arrhythmias,5) hypokalemia include lassitude and abnormalities in cardiac condition6) and hypocalcemia include tetany, numbness and cardiac dysrhythmias.7) However, mild electrolyte abnormalities are often overlooked until further worsening. Additionally, the increasing severity of adverse effects may necessitate cessation of Cmab therapy, affecting patient prognosis. Therefore, it is crucial to regularly monitor serum electrolyte levels.
A systematic review has reported the incidence of all grades Cmab-induced hypomagnesemia as 23.7%.2) A randomized-controlled trial has reported that the incidence of hypomagnesemia in patients receiving Cmab therapy was 34.9%.3) Furthermore, the onset of hypomagnesemia is known to be affected by the administration of platinum-containing drugs such as cisplatin.8,9) Meanwhile, the incidence of hypocalcemia and hypokalemia was reportedly 16.8% and 8.0-12.6%, respectively.3,4) However, in patients receiving Cmab therapy, electrolyte abnormalities, such as hypokalemia and hypocalcemia, have been reported in a limited number of studies. It is crucial to maintain the balance between these electrolytes to efficaciously continue Cmab therapy. Nevertheless, little is known regarding the relationship between these electrolytes in patients undergoing Cmab therapy. Hence, the aim of this study was to investigate the relationships between serum potassium, magnesium, and calcium levels to clarify the relationships between the onset of hypokalemia, hypomagnesemia, and hypocalcemia.
We conducted a retrospective study to investigate Cmab-induced electrolyte abnormalities at Kindai University Nara Hospital. We identified patients with unresectable advanced or recurrent colorectal cancer and head and neck cancer, treated with Cmab from September 2012 to September 2020 using medical records. We excluded patients with a history of anticancer drug therapy. Patients were administered 4 regimens: Cmab monotherapy; SOX+Cmab therapy, which included S-1, oxaliplatin, and Cmab; CBDCA+5-FU+Cmab, which included carboplatin, 5-fluorouracil, and Cmab; PCE therapy, which included paclitaxel, carboplatin, and Cmab. Demographic, clinical, and biochemical data were retrieved from the computerized hospital information system and medical records. Patient serum potassium, magnesium, and calcium levels were investigated until the end of Cmab administration or change in regimen. The serum calcium level was corrected using Payne’s equation.10) Creatinine clearance (Ccr) was calculated using the Cockcroft-Gault formula.11)
Definition of Electrolyte AbnormalitiesElectrolyte abnormalities were graded according to Common Terminology Criteria for Adverse Events version 5.0 translated by the Japanese Clinical Oncology Group (CTCAE ver. 5.0 JCOG version).12) We defined electrolyte abnormalities as follows: serum potassium level < 3.6 mmol/L, hypokalemia; serum magnesium level < 1.8 mg/dL, hypomagnesemia; serum corrected calcium level < 8.8 mg/dL, hypocalcemia. The lowest serum potassium, magnesium, and calcium levels during the period from the initiation of Cmab to the index date were defined as the minimum potassium, magnesium, and calcium levels, respectively.
Statistical AnalysisThe correlation coefficient was calculated to evaluate the relationship between minimum serum electrolytes level, including potassium, magnesium, and calcium levels. The likelihood ratio test was performed to evaluate the relationship between the onset of hypokalemia, hypomagnesemia, and hypocalcemia. The statistical analyses were conducted using JMP Pro, version 15.0.0 (SAS Institute Inc., Cary, NC, USA). A probability value of < 0.05 was considered significant.
Ethical ConsiderationIn this study, all procedures involving human participants were performed in accordance with the ethical standards of the institutional research committee and the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. This study was approved by the Ethics Committees of Kindai University Nara Hospital (Approval ID: 19-44) on April 20, 2020.
A flowchart of the patient selection procedure is presented in Fig. 1. A total of 113 patients received Cmab during the study period. We excluded 28 patients who had a history of anticancer drug therapy and 47 patients with unclear serum electrolyte levels. Additionally, 7 patient who had not completed regimen, 6 patients who had only one administration of Cmab, and 1 patient with unclear serum albumin level were excluded from this study. Patient characteristics are presented in Table 1. In total, 24 patients (21 males and 3 females) treated with Cmab were identified during the study period. In this study, the mean patient age was 72.0 ± 7.9 years. Cmab was administered for the treatment of colorectal cancer and head and neck cancer in 2 and 22 patients, respectively. In all patients, liver and renal functions remained normal. Patient treatment details are presented in Table 2. Radiation therapy was additionally administered to 21 patients. The incidence of patients receiving monotherapy and combination therapy groups was 83.3% (20/24) and 16.7% (4/24), respectively. In the combination therapy group, SOX+Cmab, CBDCA+5-FU+Cmab, and PCE were administered to 1, 1, and 2 patients, respectively.
Flowchart of the Patient Selection Procedure
| Total number | 24 | ||
| Male/Female | 21 / 3 | ||
| Age (year) | 72.0 | ± | 7.9 |
| Body Height (m) | 1.64 | ± | 0.06 |
| Body Weight (kg) | 62.9 | ± | 11.8 |
| BMI (kg/m2) | 23.3 | ± | 3.8 |
| Before administration | |||
| Alb (g/dL) | 3.9 | ± | 0.7 |
| Scr (mg/dL) | 0.9 | ± | 0.3 |
| Ccr (mL/min) | 67.9 | ± | 27.4 |
| AST (U/L) | 22.1 | ± | 6.3 |
| ALT (U/L) | 17.4 | ± | 8.0 |
| Na (mmol/L) | 139.2 | ± | 2.7 |
| K (mmol/L) | 4.4 | ± | 0.5 |
| Mg (mg/dL) | 2.2 | ± | 0.3 |
| Ca (mg/dL) | 9.4 | ± | 0.4 |
| Type of cancer | |||
| Colorectal | 2 | ||
| Head and neck | 22 | ||
Mean ± Standard deviation. BMI: Body-mass index, Alb: albumin, Scr: serum creatinine, Ccr: creatinine clearance, AST: aspartate aminotransferase, ALT: alanine aminotransferase, Na: sodium, K: potassium, Mg: magnesium, Ca: calcium.
| Combined of therapy | |
| Radiation therapy | 21 |
| Platinum-containing drugs | 4 |
| List of regimens | |
| Monotherapy | 20 |
| Combination therapy | 4 |
| SOX+Cmab | 1 |
| CBDCA+5-FU+Cmab | 1 |
| PCE | 2 |
Cmab: cetuximab, SOX: S-1 and oxaliplatin, CBDCA: carboplatin, 5-FU: 5-fluorouracil, PCE: paclitaxel, carboplatin, and Cmab.
The relationships between minimum serum levels of potassium, magnesium, and calcium are presented in Fig. 2. In all patients, significant correlation was observed between calcium and potassium (r = 0.502, p = 0.013). However, no significant correlations were observed between potassium and magnesium (r = -0.056, p = 0.796), and between magnesium and calcium (r = -0.195, p = 0.360). In the non-hypomagnesemia group, significant correlations were observed between calcium and potassium (r = 0.567, p = 0.018), between potassium and magnesium (r = 0.621, p = 0.008), and between magnesium and calcium (r = 0.508, p = 0.038). Meanwhile, in the hypomagnesemia group, no significant correlations were observed between each electrolytes. Patients who developed hypokalemia, hypomagnesemia, and hypocalcemia are presented in Table 3. Electrolyte abnormalities developed in 58.3% (14/24) of patients. The incidence of hypokalemia, hypomagnesemia, and hypocalcemia was 25.0% (6/24), 29.2% (7/24), and 25% (6/24), respectively. Among the three types of electrolyte abnormalities observed, 9 patients presented one electrolyte abnormality, and five demonstrated two abnormalities. Overall, 35.7% (5/14) of patients developed multiple electrolyte abnormalities. The incidence of hypokalemia was significantly higher patients with hypocalcemia than non-hypocalcemia (odds ratio: 16.0, 95% confidence interval: 1.7-151.1, p = 0.009). However, the incidence of hypomagnesemia was not associated with the onset of hypokalemia and hypocalcemia.
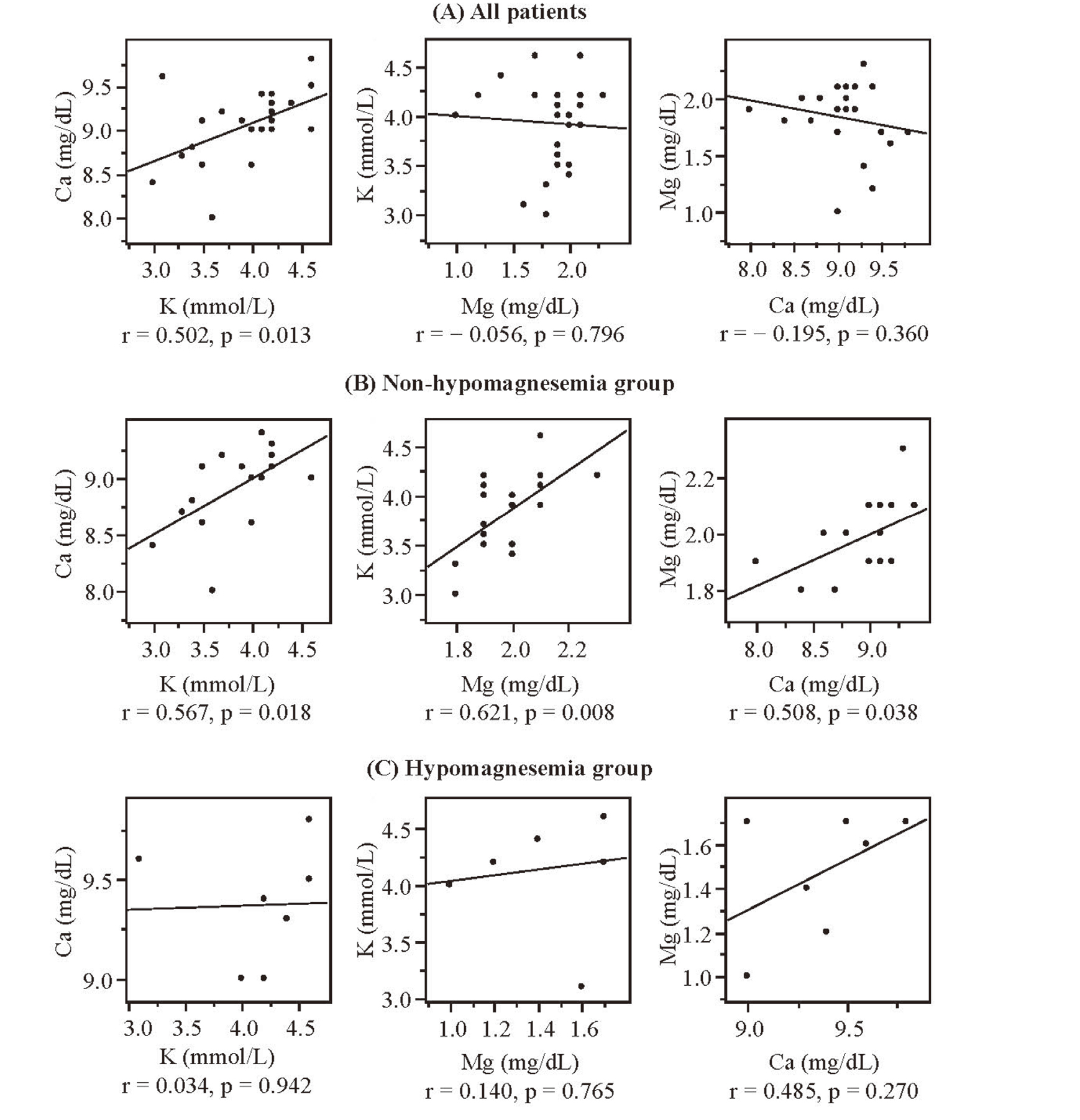
Relationships Between Minimum Serum Potassium, Magnesium, and Calcium Levels in All Patients (A), Non-Hypomagnesemia Group (B), and Hypomagnesemia Group (C)
p: statistical significance obtained using the correlation analysis. The significance level was set to 0.05. r: correlation coefficient, K: potassium, Mg: magnesium, Ca: calcium.
| Patients No. | Electrolyte abnormalities | |||
|---|---|---|---|---|
| K | Mg | Ca | Total | |
| 1 | Yes | Yes | No | 2 |
| 2 | Yes | No | Yes | 2 |
| 3 | Yes | No | Yes | 2 |
| 4 | Yes | No | Yes | 2 |
| 5 | Yes | No | Yes | 2 |
| 6 | No | Yes | No | 1 |
| 7 | No | Yes | No | 1 |
| 8 | No | Yes | No | 1 |
| 9 | No | Yes | No | 1 |
| 10 | No | Yes | No | 1 |
| 11 | No | Yes | No | 1 |
| 12 | No | No | Yes | 1 |
| 13 | Yes | No | No | 1 |
| 14 | No | No | Yes | 1 |
| 15 | No | No | No | 0 |
| 16 | No | No | No | 0 |
| 17 | No | No | No | 0 |
| 18 | No | No | No | 0 |
| 19 | No | No | No | 0 |
| 20 | No | No | No | 0 |
| 21 | No | No | No | 0 |
| 22 | No | No | No | 0 |
| 23 | No | No | No | 0 |
| 24 | No | No | No | 0 |
| n | 6 | 7 | 6 | 14 |
| % | 25.0 | 29.2 | 25.0 | 58.3 |
K: potassium, Mg: magnesium, Ca: Calcium.
In present study, we have shown that the significant correlations are observed between potassium, magnesium, and calcium in the non-hypomagnesemia group, but are not observed in the hypomagnesemia group. In the non-hypomagnesemia group, serum calcium levels significantly correlated with potassium levels. The calcium-sensing receptor (CaSR) is expressed in multiple tissues, including the parathyroid gland and kidney, and controls calcium homeostasis.13) Activation of CaSR is known to suppress the renal outer medullary potassium channel (ROMK), a potassium excretion channel from the intracellular space to the lumen,14) and calcium stimulates this receptor. Persistent hypocalcemia mitigates ROMK suppression by CaSR. As a result, potassium excretion increases. Our findings suggest that there is a positive correlation between serum potassium and calcium levels in the non-hypomagnesemia group. Additionally, it has been reported that magnesium stimulates CaSR,15) and hypomagnesemia may result in increased potassium excretion, as well as hypocalcemia.16) The present study demonstrated that there are positive correlations between serum potassium, magnesium, and calcium levels in the non-hypomagnesemia group. In renal tubules, Cmab inhibits EGFR and suppresses magnesium reabsorption mediated by transient receptor potential member 6 (TRPM6).17) These previous studies suggest that Cmab-induced magnesium deficiency may cause hypokalemia. Hypomagnesemia has been postulated to induce hypocalcemia mediated by parathyroid hormone (PTH) deficiency and peripheral resistance to PTH.18) Tsujii et al. have reported that in patients with colon cancer treated with Cmab or panitumumab therapy, serum magnesium levels positively correlated with calcium levels, and our findings are consistent with this previous study.19) Thus, these electrolytes are assumed to affect each other, and the incidences of electrolyte abnormalities may increase when these correlations are disordered.
The present study demonstrated that electrolyte abnormalities developed in 58.3% (14/24) of patients undergoing Cmab therapy, with the incidence of hypokalemia, hypomagnesemia, and hypocalcemia documented as 25.0% (6/24), 29.2% (7/24), and 25.0% (6/24), respectively. A randomized-controlled trial has reported that the incidences of hypokalemia, hypomagnesemia, and hypocalcemia were 12.6%, 34.9%, and 16.8%, respectively.3) The incidence of hypomagnesemia was lower in our study than previous study.3) This is attributed to administration of cisplatin. The cellular uptake of cisplatin is mediated via organic cation transporter 2 (OCT2) expressed at the basolateral membrane of proximal tubules,20) and may impair electrolyte reabsorption in the proximal tubules. In our study, there are no patients who were administrated cisplatin, and it is assumed that the incidence of hypomagnesemia was low. In the present study, the onset of hypokalemia was significantly associated with the onset of hypocalcemia. The majority of the body magnesium is known to present in bone, with only 1% in the blood.21) Hence, the body magnesium may be deficiency even if the serum magnesium level is within normal range. Reportedly, patients who developed hypomagnesemia occur in conjunction with hypokalemia (40-60%), and hypocalcemia (30%).22,23) Therefore, the patient who developed hypokalemia or hypocalcemia is possible to develop hypomagnesemia even if the serum level is within normal range. These results suggest that it is crucial to monitor serum levels of potassium, magnesium, and calcium during Cmab administration. Furthermore, the supplementations of multiple electrolytes are needed to treat electrolyte abnormalities.
Notably, several potential limitations should be considered while interpreting the results of this study. First, this was an observational study, with a retrospective design and a small study population. Second, we limited the patients to the initial Cmab administration, excluding patients in whom the regimen was modified as it remains necessary to evaluate electrolytes affecting Cmab therapy. However, reports examining electrolyte abnormalities with Cmab administration are limited, and it has been demonstrated that these adverse effects may be induced relatively early. Although the number of samples have to increase, these results contain useful and novel findings. Finally, the present study was not intervention study; however, our findings may provide new evidence that the management of multiple electrolytes is needed to prevent electrolyte abnormalities in patients receiving Cmab. These results highlight the importance of monitoring serum electrolyte levels in clinical settings, with further studies needed to elucidate this finding.
The present study demonstrated that the significant correlations are observed between serum potassium, magnesium, and calcium levels in the non-hypomagnesemia group, but are not observed in the hypomagnesemia group. Additionally, the incidences of hypokalemia, hypomagnesemia, and hypocalcemia were 25.0% (6/24), 29.2% (7/24), and 25% (6/24), respectively. Furthermore, the onset of hypokalemia was significantly associated with the onset of hypocalcemia. These data suggest that monitoring multiple electrolytes is important in clinical settings and plays an important role in continuing efficacious Cmab therapy.
Conflict of interestThe authors declare no conflict of interest.