2022 年 5 巻 4 号 p. 80-83
2022 年 5 巻 4 号 p. 80-83
The third vaccination for coronavirus disease 2019 is recommended; however, the vaccination rate is not increasing. This is due to a lack of information on the effectiveness of the booster vaccination and the fact that some people assume that they have acquired sufficient immunity after the second vaccination. Therefore, this study examined whether a system could be established in which a community pharmacy could act as a starting point to conduct antibody tests. The study was conducted between November 18, 2021 and February 28, 2022. Thirty-eight subjects collected their own samples in the Greenmedic pharmacy or at home using a self-blood collection kit and submitted them to the pharmacy. Samples were transported from the pharmacy to the AC clinic within 2 days for measuring anti-SARS-CoV-2 neutralizing antibodies (NAbs). There were eight men and 30 women with a mean age (mean ± standard deviation) of 33.6 ± 15.3 years. Of the subjects, 12 had completed the third vaccination; the median (interquartile range) blood NAbs after the second vaccination was 34.9 (23.3–59.4) AU/mL, which increased to 994.1 (974.7–1042.1) AU/mL after the third vaccination (p = 0.0022). Significant negative correlations were also observed for both blood NAbs and age after the second vaccination (rs = −0.579, p = 0.00014). Since these results were similar to those of previous studies conducted at hospitals, this study seems to be the first to demonstrate that anti-SARS-CoV-2 neutralizing antibody test flow can be established at a community pharmacy.
The third vaccination for the coronavirus disease 2019 (COVID-19) (booster vaccination) has been recommended by the Japanese government to prevent severe cases of COVID-19 infection and to normalize social and economic activities in the era of the novel coronavirus; however, the current vaccination rate is not increasing. These are attributed to concerns about vaccine adverse reactions and individual negligence. Particularly, the lack of information on the effects of booster vaccination and the fact that some people assume without evidence that sufficient immunity has been acquired through the two vaccinations lead to nonchalance. Therefore, it is important to provide accurate information and create an environment in which people can correctly understand their immunity.
To correctly measure one’s own antibody titer, one can visit a hospital where antibody testing is available or a test kit can be purchased via the Internet and conducting the test at home. However, going to a specific hospital for testing is labor-intensive and may increase the risk of infection. In addition, it is often difficult for the elderly and others who are unfamiliar with the Internet to purchase and use test kits without support.
However, community pharmacies are more numerous than convenience stores1) and since they are medical institutions that anyone can use, they are expected to play a central role in community healthcare. In fact, the “Vision of pharmacy for patients—From front-door to family and community”2) formulated by the Ministry of Health, Labor and Welfare clearly states that pharmacies should appropriately provide health-related consultations to local residents and, if necessary, recommend that they visit a medical institution or undergo a medical checkup. In this study, we examined the possibility of establishing a system in which a community pharmacy can take the initiative in conducting anti-SARS-CoV-2 neutralizing antibodies tests to realize safe and secure community medical care during the novel coronavirus era.
Health care workers belonging to the Greenmedic pharmacy and AC clinics, who gave their written consent to participate in the study, were included in the study. Subjects who had not received the COVID-19 vaccine were excluded from the study. The study was conducted from November 18, 2021 to February 28, 2022.
Anti-SARS-CoV-2 Neutralizing Antibody Assay FlowThe flow of the anti-SARS-CoV-2 neutralizing antibodies (NAbs) measurement is shown in Fig. 1. Participants collected their own samples at the Greenmedic pharmacy or at home using a home-based self-blood collection test kit (Self Dock Club®, Health Wave Japan) and submitted them to the Greenmedic pharmacy. The subjects used a sterile puncture device (lancet) in the test kit to collect a small amount of blood (0.3 mL) from their fingertips in a microtube with a separating medicine. The collected blood was inverted and mixed in microtubes, kept in a cool and dark place in a double-packed container, and transported from the Greenmedic pharmacy to the AC clinic laboratory within 2 days. Samples were collected approximately 6 months after the second vaccination with the COVID-19 vaccine (COMIRNATY intramuscular injection, Pfizer) and 1–3 weeks after the third vaccination. At the AC clinic laboratory, samples were measured using a fully automated chemiluminescent immunoassay (CLIA) device (iFlash1800, Shenzhen YHLO Biotech Co.) and anti-SARS-CoV-2 NAbs test reagent (iFlash-2019-nCoV NAbs assay, Shenzhen YHLO Biotech Co.) Blood samples in microtubes were centrifuged at 2,000 × g for 5 min to ensure a minimum of 48 µL to 100 µL of serum, and then placed in a CLIA device. The neutralizing capacity of antibodies was estimated using the SARS-CoV-2 surrogate virus neutralization test. The iFlash-2019-nCoV NAbs assay is a one-step competitive paramagnetic particle chemiluminescent immunoassay for the quantitative measurement of 2019-nCoV NAbs in human serum. The assay detects NAbs that inhibit the binding of the binding site (RBD) to angiotensin-converting enzyme 2 (ACE2); therefore, the presence of a neutralizing antibody in the samples inhibits the RBD-ACE2 interaction and thus decreases the luminescence value. By automatically generating a calibration curve with a 4-point calibrator, the measurement results are reported as inhibition activity (AU/mL), where < 10.0 AU/mL is considered negative and ≥ 10.0 AU/mL is considered positive (according to the manufacturer’s information).

Anti-SARS-CoV-2 NAbs Measurement Flow Starting from the Pharmacy
Pharmacy Site
1. Explanation of research and obtaining consent
2. Collection of samples by the subjects themselves and submit them to the pharmacy
3. Samples are transported to the clinic under room temperature in strict packaging
Clinic site
4. Receive samples and measure NAbs
5. Feedback of NAbs measurement results
The subjects’ age, sex, body mass index (BMI), date of the second vaccination with the COVID-19 vaccine, highest body temperature after the vaccination, and date of the third vaccination were collected by administering a Google Form questionnaire to the subjects.
Statistical AnalysisSpearman’s correlation analysis was performed to determine the correlation between NAbs after the second vaccination with the COVID-19 vaccine and age, BMI, and maximum body temperature after vaccination. In addition, the Wilcoxon signed-rank test was used to compare the NAbs after the second and third vaccinations.
Ethical ConsiderationsThe study protocol was in accordance with the Declaration of Helsinki. Approval was obtained from the Japanese Association for Community Pharmacy Ethics Review Committee prior to the collection of data and samples (approval number:202103).
There were 38 subjects (8 men and 30 women), with a mean age (mean ± standard deviation, SD) of 33.6 ± 15.3 years. Twelve of the subjects had completed their third vaccination, of which 10 were vaccinated with the COMIRNATY intramuscular injection and two with the Spikevax intramuscular injection (Moderna). The mean elapsed time (mean ± SD) from vaccination to sample collection was 183.0 ± 15.4 and 12.9 ± 2.5 days after the second and third vaccinations, respectively.
Comparison of Blood NAbs after the Second and Third COVID-19 VaccinationThe median (interquartile range) blood NAbs after the second vaccination was 34.9 (23.3–59.4) AU/mL, which increased to 994.1 (974.7–1042.1) AU/mL after the third vaccination) (p = 0.0022) (Fig. 2). When the cutoff value for blood NAbs was set at 10 AU/mL based on the manufacturer’s information on the anti-SARS-CoV-2 neutralizing antibody test reagent, 11 out of 12 (91.7%) exceeded the cutoff value after the second immunization, but all 12 (100%) exceeded the cutoff value after the third immunization.

NAbs after 2nd vs. 3rd Vaccination (n = 12)
Significant negative correlations were found for both blood NAbs and age and blood NAbs and BMI (age: rs = −0.579, p = 0.00014; BMI: rs = −0.4301, p = 0.0070, Fig. 3A, 3 B). Conversely, there was a trend toward a weakly positive, although not significant, correlation between blood NAbs and maximum body temperature (rs = 0.3063, p = 0.0781, Fig. 3C).
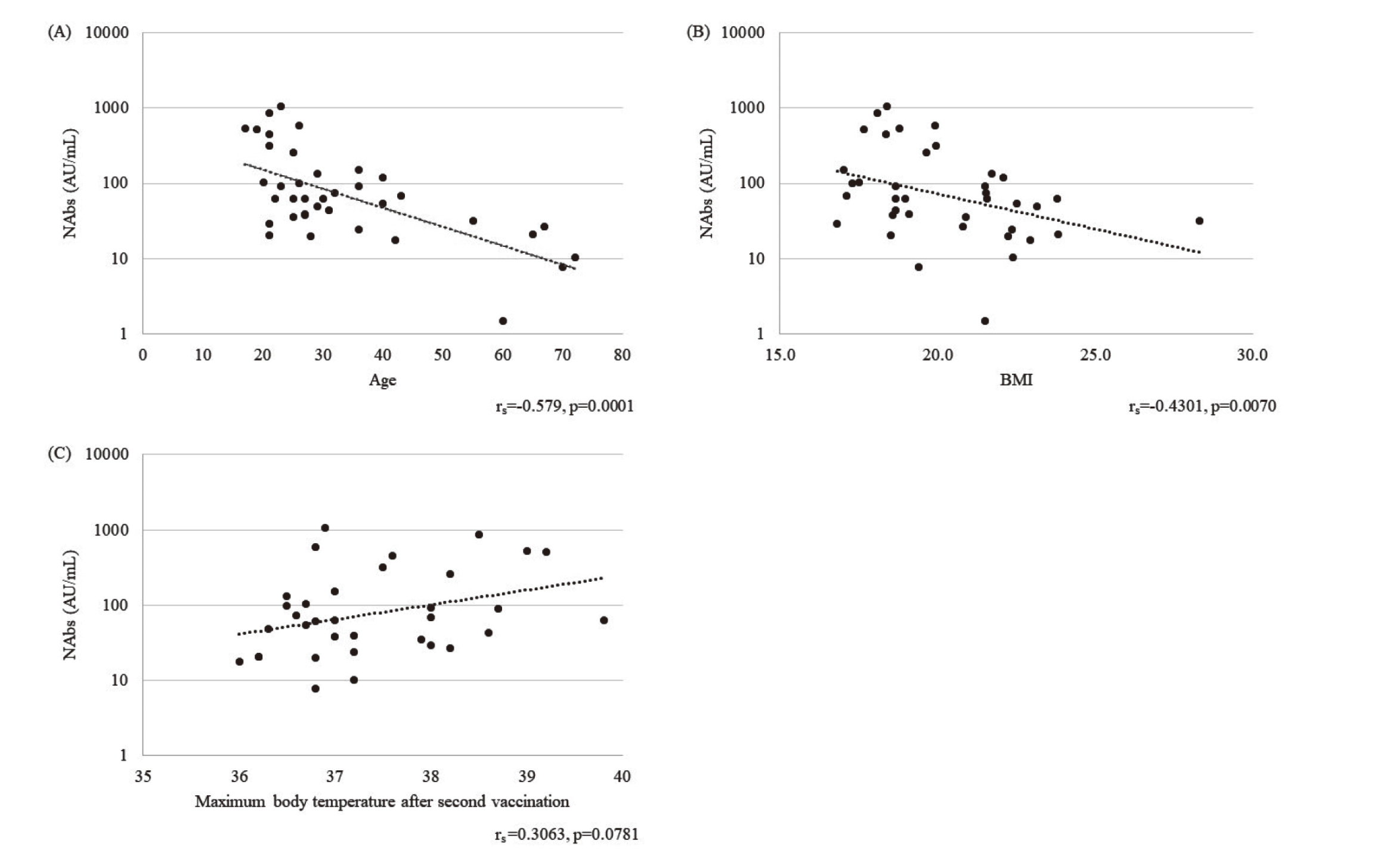
Correlation between NAbs and Backgrounds of Subjects, (A) Age (n = 38), (B) BMI (n = 38), (C) Maximum Body Temperature after Second Vaccination (n = 34)
In our study, the blood NAbs level after the third vaccination was 38.9 times higher than that after the second vaccination. Furukawa et al.3) reported at Kobe University Hospital that the neutralizing antibody titer against the Omicron variant after the third vaccination was 39 times higher than that at 7 months after the second vaccination. Although our study did not calculate antibody titers, making the direct comparison with the previous study difficult, we support the report by Furukawa et al.3) in that higher levels of neutralizing antibodies were produced after the third vaccination than after the second vaccination. In addition, although an effective neutralizing antibody concentration is defined as 10 AU/mL, Nakano et al.4) reported that neutralizing antibody levels in COVID-19 patients exceeded 10 AU/mL after 15 days of disease onset. Furthermore, Favresse et al.5) reported higher NAbs in vaccinated patients than in previously infected patients after a second vaccination. In the present study, measurements were taken at an average time point of approximately 13 days after the third vaccination, and all subjects had antibody concentrations above 10 AU/mL. Based on the above, we believe that the effectiveness of the third vaccination was confirmed even with an antibody testing system originating from a community pharmacy, although no direct comparative study has been conducted.
In our study, the correlation between blood NAbs after the second vaccination and the subjects' age, BMI, and maximum body temperature showed a significant negative correlation with age and BMI. However, several previous studies6–8) have reported a negative correlation between blood NAbs and age at second vaccination. In addition, Popkin et al.9) conducted a systematic review of the association between obesity and COVID-19 and reported that obesity was significantly associated with increased morbidity and mortality of COVID-19 and that obesity may weaken the effect of vaccination. The results obtained in this study support those of the previous studies.
In contrast, there was a trend toward a weak but not significant positive correlation between blood NAbs and maximum body temperature after the second vaccination. Furukawa et al.3) reported no correlation between body temperature and anti-SARS-CoV-2 neutralizing antibody titers after vaccination. However, Tani et al.6) reported that the higher the maximum body temperature after the second vaccination, the greater the blood NAbs levels. Although the small number of subjects in this study compared to those in these studies may have prevented a significant difference, the trend seems to support the study by Furukawa et al.3). The importance of booster vaccination against the Omicron variant is increasing, as the second vaccine was insufficiently effective, whereas the third vaccination induced high levels of neutralizing antibodies regardless of age.3) Moreover, Favresse et al.5) reported that vaccinated persons showed higher antibody titers than previously infected persons, which suggests that it is important to have a system that allows easy implementation of anti-SARS-CoV-2 neutralizing antibody testing because it can sound a warning bell with facts against the unfounded assumption that “I may have been infected without realizing it, so I should be fine.”
The limitations of this study are that the number of cases was small and general pharmacy users were not included; therefore, it will be necessary to include the general public in future studies and to evaluate the simplicity of this antibody testing flow starting from the pharmacy using questionnaires. However, this study is the first in Japan to demonstrate with objective numerical values that a simple anti-SARS-CoV-2 neutralizing antibody test flow can be established using a community pharmacy as the starting point, and we believe that this is a valuable finding that could serve as a trigger for expanding the functions of community pharmacies in the novel coronavirus era.
Conflict of interestThe authors declare no conflict of interest.