2022 年 5 巻 4 号 p. 88-93
2022 年 5 巻 4 号 p. 88-93
The cysteine protease papain was shown to induce production of IL-4 and other cytokines in murine basophils, a critical event for initiating and promoting type 2 immune responses. However, the papain ‘sensor(s)’ and the intracellular signaling pathway for IL-4 production triggered by the protease remained unknown. Here we show that basophils lacking FcRγ or expressing dominant negative Syk failed to produce IL-4 in response to papain, indicating the involvement of the FcRγ-Syk pathway that was essential also for IL-3-induced IL-4 production. While IL-3, IgE cross-linkage and papain induced production of IL-4, IFN-β production was induced only by papain, indicating that papain sensor(s) or its downstream signaling pathways for production of IL-4 seemed to be distinct from those for IFN-β production. We further showed using the artificial papain sensor CD8-FcRγ fusion protein that IL-3 potentiated, independently of FcRγ, basophil response to FcRγ-dependent papain signals. Thus, our current study showed that papain sensing and/or downstream signaling are unique for cytokines to be induced and regulated through cross-talk with other signals such as IL-3 signals.
It is well established that proteases, regardless whether endogenously or exogenously derived, exhibited a wide variety of effects on immunity. Among these immune-modulating activities of proteases, particularly interesting was those on the type 2 immunity since type 2 immune responses were considered important in protective immunity against helminths and allergic inflammation caused by these proteases.1–3) It has been reported that proteases secreted by multicellular helminths and protease-allergens derived from organisms such as rag weed pollen, cockroach, Aspergillus and house dust mites induced type 2 immunity uniquely.4,5) As for a mechanism of protease-induced inflammation, the cellular G-protein-coupled receptor protease activating receptor 2 (PAR2), expressed on mucosal epithelial cells, was shown to recognize serine and cysteine proteases and induce the release of pro-inflammatory mediators including IL-6, IL-8, TNF-α and thymic stromal lymphopoietin (TSLP), thereby implicated in allergic inflammations6–11). Proteases may induce type 2 immune responses also through triggering the production of the Th2 cytokine IL-4. Thus, it has recently shown that the cysteine protease papain induced IL-4 production by basophils,12) rare blood cells that were shown to infiltrate into inflamed tissues and produce IL-4 upon helminth infection and administration of allergens.13–19) Although recently established genetically modified mice, in which basophils could be depleted constitutively or inducibly in vivo, questioned the essential roles for basophils in the initiation of Th2 responses,20,21) IL-4 derived from basophils appeared to contribute to Th2 responses under certain conditions.20,22) Consistent with this notion, spontaneous or induced basophilia resulted in excess Th2 differentiation in vivo.23–25) Although bone marrow (BM) -derived basophils (BMBs) indeed produced IL-4 in vitro upon stimulation with papain in a manner dependent on its protease activity, basophils did not express PAR2,12) and the mechanism remains unaddressed as to how basophils ‘sense’ the protease activity, what kind of intracellular IL-4-inducing signaling pathway(s) are activated upon protease sensing and how these signaling pathways are regulated.26)
Large numbers of studies have shown that human and murine basophils produced ample amounts of IL-4 in response to a variety of physicochemically different stimuli in addition to those that crosslinked IgE associated with FcεRI.26–29) Such stimuli include cytokines such as IL-3, IL-18 and IL-33.30–32) The intracellular signals triggered by those substances seemed to be transduced mainly via two pathways, the immunoreceptor tyrosine-based activation motif (ITAM)-Syk and MyD88-NF-κB pathways.31,33,34) Here we found that FcRγ, an adaptor molecule containing an ITAM critical for signaling via FcεRI and other immunoreceptors,35) played critical roles in the production of IL-4 in response also to papain in basophils in vitro. Furthermore, we also found that IL-3 not only induced IL-4 production in an FcRγ-dependent manner but also potentiated FcRγ-dependent papain-induced signals in an FcRγ-independent manner. These results collectively indicated that basophils ‘sensed’ external signals such as IL-3 and papain with multiple surface molecules and such surface molecules triggered distinct intracellular signaling leading to the production of multiple cytokines.
All mice used in this study were maintained in the animal facilities in Nagoya city University and/or Shinshu University under strictly controlled specific pathogen-free conditions, with regular monitoring of infection, and used at 8–14 weeks of age. FcRγ−/− mice on the C57BL/6 background were described previously.31) C57BL/6 mice were from SLC (Shizuoka, Japan). Control mice for FcRγ−/− mice were in most cases littermates heterozygous for the genes that gave results undistinguishable from those obtained using wild-type mice. All animal experiments were approved by the Committee for Animal Experimentation and Care of Nagoya City University and Shinshu University and conducted according to the guideline.
Cell CultureBM-derived basophils (BMBs) were obtained as described24) with slight modification. Briefly, whole BM cells (2 x 107) were cultured in 10 mL of 10% fetal bovine serum-containing RPMI1640 medium (Nissui, Japan) supplemented with recombinant murine IL-3 (5 ng/mL) for 11–12 days with medium changed every 3 days. Recombinant murine IL-3 was produced by a cell line transfected with murine IL-3 expression vector (a kind gift from Prof. Hajime Karasuyama). BM-derived basophils were purified by depleting mast (c-kit+) cells using IMag beads (BD Biosciences), and used for stimulation. BMBs were stimulated with IL-3 (5 ng/mL), anti-CD200R3 antibody (0.5 μg/mL), anti-DNP IgE mAb (SPE-7, SIGMA or Nihon biotest, 1 μg/mL) +anti-IgE Abs (BD Biosciences, 1 μg/mL) or anti-DNP IgE mAb (2 or 5 μg/mL) +DNP-BSA(2 or 5 μg/mL), and papain (Calbiochem, 100 μg/mL). For IFN-β pretreatment, BMBs were washed extensively, depleted of c-kit+ mast cells, seeded at 1 or 2 x 106/mL and incubated for 20–24 h. The supernatants were collected to measure IL-4 proteins using Mouse IL-4 ELISA antibodies set (Biolegend). RNA was isolated from BMBs using RNAiso plus reagents (Takara bio). RT-PCR was carried out on cDNA prepared using PrimeScript RT Master Mix (Takara bio) on a Thermal Cycler Dice (Takara bio). The sense and antisense primer sequences were 5’-AGATGGATGTGCCAAACGTCCTCAC-3’ and 5’-AACGACCTTGGAAGCCCTACAGAC-3’ for mouse IL-4 ; 5’-GCTCCTGGAGCAGCTGAATGG-3’ and 5’-TCTCTTGGATGGCAAAGGCAGT-3’ for mouse IFN-β; 5’-TGAACCCTAAGGCCAACCGTGA-3’, and 5’-CCTGTGGTACGACCAGAGGCATAC-3’ for mouse β-actin.
Retroviral InfectionRetroviral vectors were used for transfection of FcRγ and its mutants into BMBs.31) These retroviral constructs were transiently transfected into the packaging cell line Phoenix using GeneJuice reagent (Merck Millipore). Retrovirus-containing supernatants were collected 48 h after transfection, concentrated about 10-fold by centrifugation and added to 12-well plates, which were processed by sequential treatment with RetroNectin solution (20 μg/mL, Takara Bio) in PBS. After incubating the plates with the virus supernatants for 3 h at room temperature, BMBs (1 x 106 cells/mL) were added and infected for 2 days. Cells stably expressing ratCD2 were sorted with IMag-beads, and used for stimulation.
Statistical AnalysisStatistical significance was calculated with Mann-Whitney U-test.
BMBs produced IL-4 upon stimulation with papain in vitro (Fig. 1A) in agreement with a previous report.12) In contrast, freshly isolated basophils did not produce detectable IL-4 under the same condition (data not shown). We found that BMBs obtained from mice lacking FcRγ (FcRγ–/– mice) were unable to produce IL-4 upon stimulation with papain (Fig. 1A). Basophils derived from these mice did not express surface FcεRI but were otherwise normal in development, IL-3-induced proliferation and IL-4 production in response to FcRγ-independent stimuli such as CD200R3 crosslinkage that signaled through another ITAM-containing adaptor, DAP-1236) (Fig. 1A). In addition, real-time PCR revealed the elevation of IL-4 messages in papain-stimulated wild-type but not FcRγ–/– BMBs (Fig. 1B). When we treated papain with the protease inhibitor E64 before addition to the cultures, the elevation of IL-4 mRNA was no longer observed (Fig. 1B). We noted that the amounts of IL-4 induced by papain were around ten times lower than those produced in response to CD200R3 or FcεRI cross-linkage (Fig. 1A). Consistently, IL-4 mRNA in these BMBs were ten-fold less relative to those in IgE-IC-stimulated BMBs (Fig. 1B). Such an inefficient IL-4 production might suggest that the intracellular signals elicited by papain were weaker than those triggered by CD200R3 or FcεRI cross-linkage. Alternatively, papain itself or the constituents of the papain preparation might be toxic to BMBs as only a small number of BMBs could be recovered 24 h after papain stimulation. Whichever the reason was, the current observations clearly indicated that BMBs ‘sensed’ the protease activity of papain with a certain FcRγ-associated surface molecule(s) for IL-4 production in agreement with a previous report.37)

Papain-Induced IL-4 Production by Bone BM-Derived Basophils Required FcRγ
BMBs derived from either FcRγ+/− (white bar) or FcRγ−/− (gray bar) mice were stimulated with papain. (A) BMBs were enriched and stimulated with medium(-), IL-3, papain, IgE+anti-IgE immune complex (IgE-IC) or anti-CD200R3 antibody (0.5 μg/mL) for 24 h, and the amounts of IL-4 produced are shown. As a positive control, 0.5 μg/mL of anti-CD200R3 antibody was used. Mean values of triplicate determinations ± SD of cytokine concentrations were measured in the supernatants using specific ELISAs. (B) RNA was extracted from the cells 6 h after stimulation, and IL-4 mRNA was measured by quantitative real-time PCR. Results are plotted as the fold increase in mRNA relative to the level determined in unstimulated cells. Error bars represent the mean ± SD, and representative of two (B) or more than three (A) independent trials.
It was shown previously that the ITAM in FcRγ and downstream Syk activation played essential roles in IL-4 production induced by IL-3 as well as FcεRI cross-linkage in basophils.31) We next examined if this intracellular signaling pathway was involved also in the signal transduction for IL-4 production triggered through the FcRγ-associated papain ‘sensors’. As shown in Fig. 2, papain-induced IL-4 production by FcRγ–/– BMBs could be restored by retrovirus-mediated expression of wild-type FcRγ but not a mutant FcRγ with the ITAM tyrosine residues replaced with leucines (Fig. 2A). Furthermore, a dominant negative Syk mutant lacking the kinase domain31) suppressed IL-4 production induced by papain as well as upon FcεRI cross-linkage, by wild-type BMBs when introduced retrovirally (Fig. 2B). These results indicated that papain stimulated IL-4 production through triggering the ITAM-Syk signaling pathway similarly as did FcεRI cross-linkage stimulation.
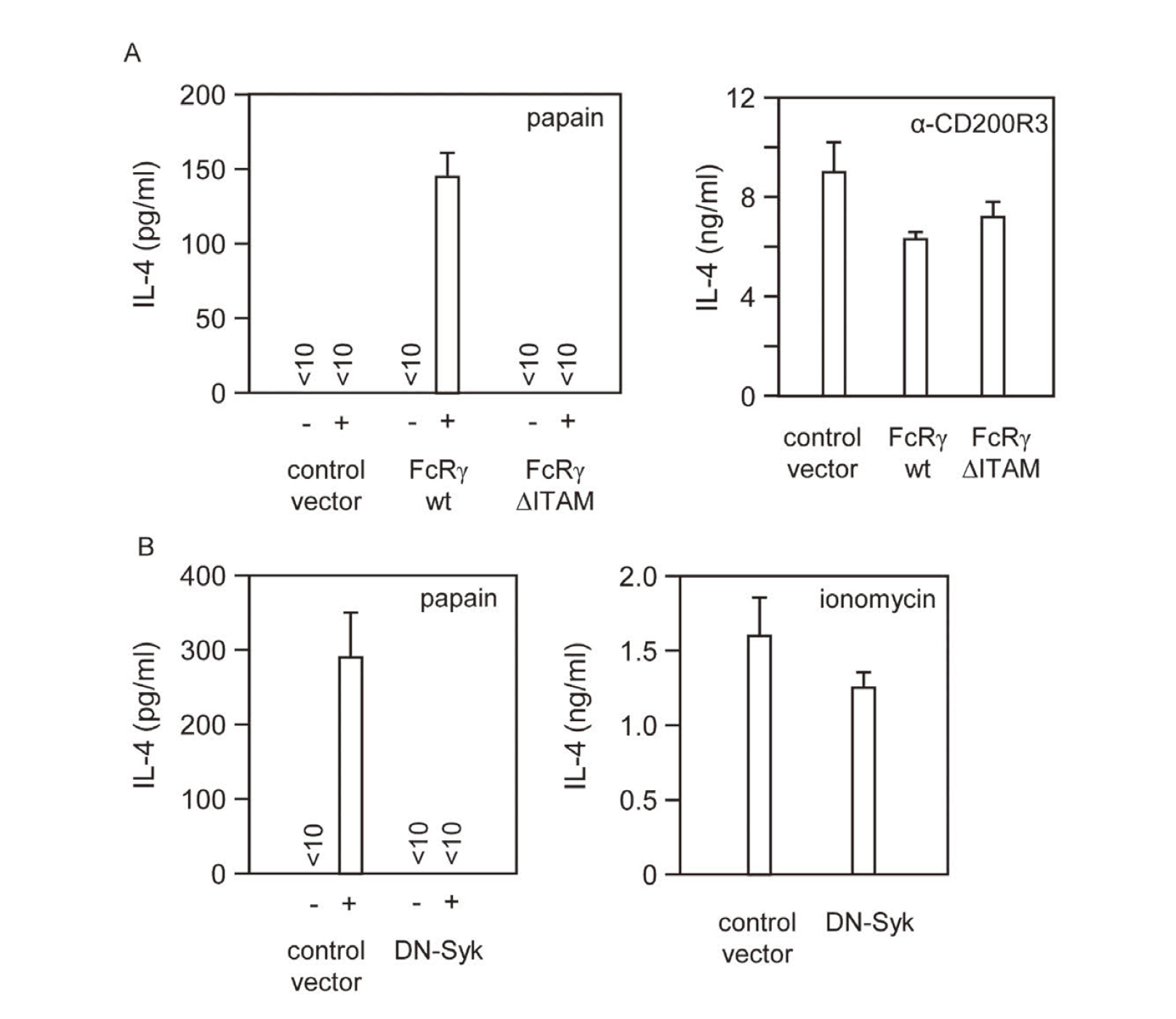
Papain Signaled through the ITAM-Syk Pathway for IL-4 Production
(A) Wild-type (WT) and mutant FcRγ (ΔITAM) with two tyrosine residues in the ITAM replaced with phenylalanine inserted into the retroviral vectors. Control represents those transduced with control vector. IL-4 production by FcRγ-deficient BMBs expressing none (control), wild-type FcRγ (WT) or FcRγ (ΔITAM) containing 60, 62 or 71% of ratCD2+c-kit−, respectively. (B) Wild-type BMB cells were retrovirally expressed with dominant-negative Syk mutant (DN-Syk) lacking the kinase domain (31), and stimulated with papain (100 μg/mL) for IL-4 production. Bars, the means and SD of duplicated cultures. Representative of three independent experiments.
Type I IFN is induced by exogeneous pathogens, such as viral and bacterial infections, and acts as a defense against infection with a wide variety of IFN-inducible genes.38,39) We investigated whether IFN-β is also produced by exogeneous cysteine proteases such as papain. We found that BMBs induced IFN-β mRNA upon stimulation with papain in a FcRγ-independent manner (Fig. 3A and B). Interestingly, unlike IL-4 mRNA, IFN-β mRNA was not induced by stimulation with IL-3 or IgE-IC, suggesting that IFN-β induction was triggered through different ‘sensor’ than that led to IL-4 production. It has been shown that FcRγ is essential for both papain- and IgE-IC-stimulated IL-4 production (Fig. 1). Interestingly, IFN-β treatment partially suppressed IL-4 production by papain but not by IgE-IC. (Fig. 3C). Therefore, it is unlikely that the genes induced by IFN-β repressively regulates the FcRγ-ITAM-Syk pathway.
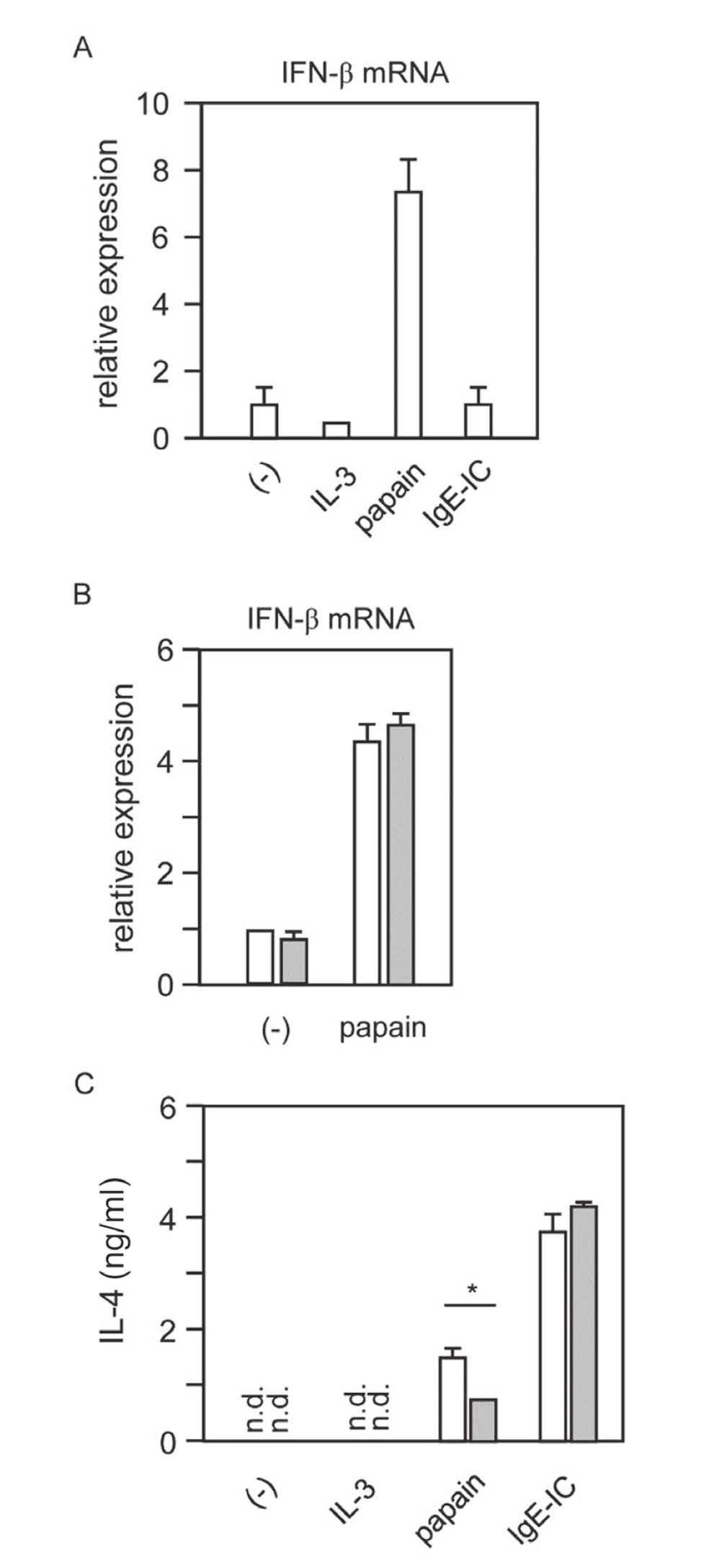
Papain Induces IFN-β in an FcRγ-Independent Manner
(A) RNA was extracted from the cells 6 h after stimulation, and IFN-β mRNA was measured by quantitative real-time PCR. Results are shown as the fold increase in mRNA relative to the level determined in unstimulated cells. (B) BMBs derived from either FcRγ+/− (white bar) or FcRγ−/− (gray bar) mice were stimulated with papain. IFN-β mRNA expression was measured under the same conditions as in A. (C) BMBs were incubated with IFN-β (20 ng/mL) for 24 h, and then washed and stimulated with IL-3 (5 ng/mL), papain (100 μg/mL), or anti-DNP IgE mAb (5 μg/mL) +DNP-BSA (5 μg/mL). IL-4 in the culture supernatant was measured by ELISA. Error bars represent the mean ± SD. Data shown are representative of three independent experiments. *p < 0.05 compared with IFN-β untreated cells.
A chimeric protein consisting of the intracellular portions of FcRγ fused to the extracellular and transmembrane portions of CD8, a molecule normally absent in basophils,40) was introduced into BMBs derived from FcRγ–/– mice. The fusion protein was expected to be the only molecule bearing the FcRγ ITAM in these BMBs and unable to associate with the molecules that normally associated with FcRγ since the transmembrane portion was derived from CD8. Consistently with this prediction, FcRγ–/– BMBs expressing the fusion protein remained to display no surface FcεRI (Fig. 4A), which was known to be transported to the cell surface only when associated with FcRγ. To our surprise, these BMBs produced low but detectable amounts of IL-4 upon stimulation with papain (Fig. 4B). As whole CD8 molecules did not confer FcRγ–/– BMBs papain responsiveness (data not shown), these observations indicated that extracellular portion of CD8 molecules could act as a papain ‘sensor’ relaying the papain signals to the intracellular portion of FcRγ. It should be noted, in addition, that IL-3 augmented papain-induced IL-4 production by FcRγ–/– BMBs expressing the fusion protein (Fig. 4C). The IL-3 signal(s) for such an augmentation appeared thus to be FcRγ-independent and distinct from the IL-4-inducing signal that was shown to be strictly dependent on FcRγ31).
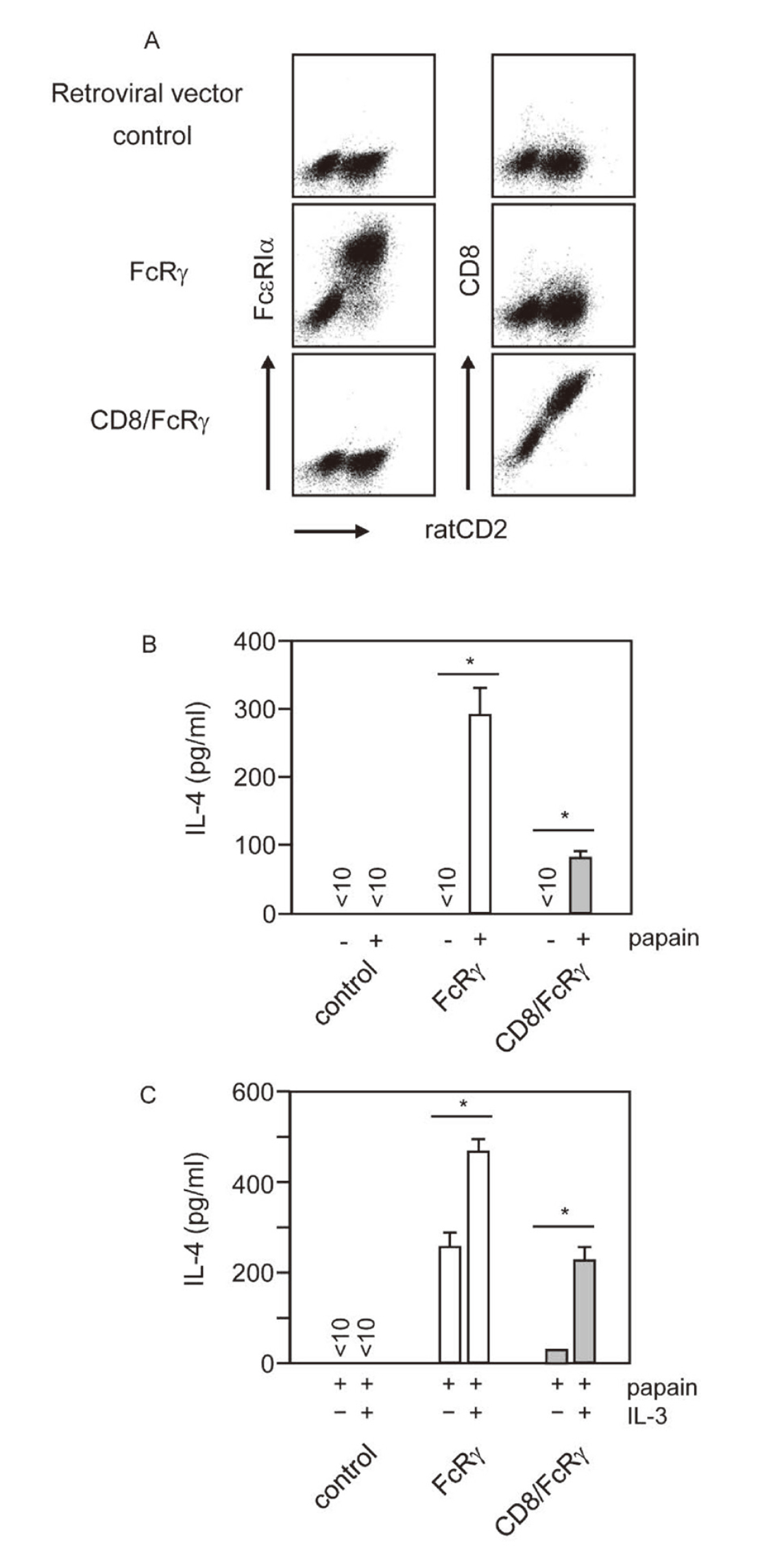
The Extracellular Portion of CD8 Molecules Fused with FcRγ Could Transduce Papain Signals
Wild type FcRγ and chimeric protein consisting of the intracellular portions of FcRγ fused to the extracellular and transmembrane portions of CD8, mutant FcRγ (CD8/FcRγ) inserted into the retroviral vectors. Control represents those transduced with control vector. (A) Surface FcεRIα and CD8 expression in FcRγ-deficient BMBs transduced with the retrovirus vectors (ratCD2+c-kit−). (B) IL-4 production by FcRγ-deficient BMBs expressing none (control), wild-type FcRγ (FcRγ), or CD8/FcRγ-chimera receptor (CD8/FcRγ) containing 77, 72 or 84% ratCD2+c-kit−, respectively, in response to papain. *p < 0.05 compared with control. (C) BMBs retrovirally expressing FcRγ or CD8/FcRγ chimeric receptor were stimulated with papain with or without IL-3, and IL-4 in the culture supernatant was measured by ELISA after 24 h. Data shown are representative of three independent experiments. Data are shown as means ± SD. Similar results were obtained in three independent experiments. *p < 0.05 compared with the results of papain alone stimulation.
In this study, we demonstrated that FcRγ-associated surface molecules ‘sensed’ the protease activity of papain and transduced signals for IL-4 production via the FcRγ ITAM and downstream Syk. Papain ‘sensing’ might not require specific structures as even CD8 molecules could mediate papain stimulation when fused with FcRγ cytoplasmic portion, although we could not exclude the possibility that endogenous papain ‘sensors’ resemble the CD8 molecule structurally, In this regard, the specific ‘ligand-receptor’ concept may not be applicable to the basophil ‘sensing’ the protease allergens. Also highlighted in this study was the versatile function of FcRγ as a common component of multiple ‘sensors’ for structurally and functionally diverse extracellular substances including IL-3,31) papain (this study), IPSE-alpha29) or IgE-antigen complexes that commonly stimulate basophils to produce IL-4. It is highly interesting to know if the FcRγ-mediated signaling pathway is also involved in basophils responses to other protease allergens such as Der p1 from house dust mites.
We observed papain-induced IL-4 production in BMBs but not in freshly isolated basophils (data not shown). This may mean that during the expansion in IL-3-supplemented cultures, basophils acquired competence to the papain signals. Since even freshly isolated basophils expressed FcεRI, it is likely that the competence was related to intracellular alterations.31) IL-3 was a cytokine produced by activated T cells and required for basophil migration to the inflamed tissues.41) As we showed in this study, IL-3 had two roles, distinguishable with respect to FcRγ-dependence, in IL-4 production by basophils. Thus, IL-3 not only triggered IL-4 production but also potentiate papain signaling for IL-4 production. In this regard, IL-3 is a versatile cytokine playing important roles in type 2 inflammation through activating multiple functions associated with basophils.
We would like to thank Drs. Takashi Saito, Hajime Karasuyama, Toshio Kitamura and Sho Yamasaki for providing us with FcRγ–/– mice, anti-CD200R3 antibody, the original pMX vector and the vector for expressing the CD8/FcRγ fusion protein, respectively. This work has been supported in part by Grants-in-Aid for from Institute of Drug Discovery Science, Nagoya City University, Japan, and JSPS KAKENHI grant number [20K07017], and grants from Takeda foundation, and the Ichiro Kanehara foundation.
Conflict of interestThe authors declare no conflict of interest.